
Nitric oxide (NO) is an essential signaling molecule and is known to play a significant role in a multitude of physiological systems including the central nervous system (CNS), the cardiovascular system, the gastrointestinal tract, the immune system, and the renal system. 1-5 However, being highly reactive, detection and quantification of NO is very difficult.6,7 It requires a sensor that is sensitive, selective to NO, and easy to calibrate.
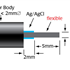
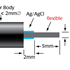
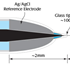
WPI’s Free Radical Analyzer (4-channel TBR4100 and single-channel TBR1025) and the LabTrax Data Acquisition System with the options of using nano NO sensors, micro NO sensors, flexible micro sensors, and macro nitric oxide sensors enable amperometric (electrical based) detection of NO dissolved in liquids.
Features & Benefits of the Free Radical Analyzer and Nitric Oxide Detection System
- Rapid response time: < 5 sec enhances detection capability of the highly reactive, short-lived NO molecules
- High sensitivity: ≤2 pA/nM – enables detection of low levels of NO
- Excellent selectivity to NO: NaNO2 (10-6 or better) prevents contamination with other isoforms
- Nano and microsensors & macrosensors are available:
- Nano and microsensors for measurement of tiny volume or space application
- Macrosensors with permeable sleeve to prevent protein deposition and interference from other constituents
- Every sensor is quality tested and sold with a calibration certificate
- Simple and easy calibration and detection method
- User friendly software for data recording (Labtrax)
- Numerous citations in the literature for detection in biological tissues, cell cultures and liquids.
Tips for Efficient and Accurate Detection
- Make fresh calibration solutions. Calibration solution, such as SNAP can be stored at 4°C for 1 week away from light (covered in aluminum foil).
- When precise detection is critical, perform daily calibrations before measuring unknown samples.
- Changes in temperature and pH are known to affect the electrical read outs. Therefore, calibration should be performed at the same temperature and pH as the sample.
- Follow the long-term and short-term storage instruction for maintaining functional longevity of the sensor.
- All the sensors have a detection range (concentration) posted in the website.
- Determine from literature the expected concentration of nitric oxide in your sample (e.g., produced in cell culture by specific cell type) then select the sensor that can detect the lowest concentration. If the sample concentration is high, it is possible to bring it within the detection range by diluting the sample.
WPI’s Free Radical Analyzer (4-channel TBR4100 and single-channel TBR1025) and the LabTrax Data Acquisition System can also be used for detection of other free radicals, including pH, oxygen (O2), hydrogen peroxide (H2O2), carbon dioxide (CO2), and hydrogen sulfide (H2S).
For more information contact WPI Technical Support or one of our Field Applications Specialist.
References
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766.
- Loscalzo, J.; Welch, G. Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 1995, 38,87–104.
- Lanas, A. Role of nitric oxide in the gastrointestinal tract. Arthritis Res. Ther. 2008, 10, S4.
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916.
- Mount, P.F.; Power, D.A. Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiol. 2006,187, 433–446.
- Butler, A.R.; Flitney, F.W.; Williams, D.L.H. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: A chemist’s perspective. Trends Pharmacol. Sci. 1995, 16, 18–22.
- Gaston, B. Nitric oxide and thiol groups. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 323–333.

















Request
Catalogue
Chat
Print