

MICRO-ePORE
Pinpoint Cell Penetrator for targeted microinjection
- Overview
- Specifications
- Accessories
- Citations
- Related Products
Overview

.png)



There are 5 images available to view - click to enlarge and scroll through the product gallery.
MICRO-ePORE Brochure
/ Download as PDF
Instruction Manual
/ Download as PDF
Quick Start Guide
/ Download as PDF
Is a reduction in embryo lysis possible using the MICRO-ePORE W.Gardiner, J.Kenyon, T.Bell, S.Atkins
/ Download as PDF
Pinpoint Cell Penetrator for targeted microinjection and increased viability of injected embryos
- Touch-screen display–resistive touch panel for use with gloves
- Injection control through foot switch or manually through touch screen
- Intuitive user-interface
- User adjustable frequency and voltage through touch screen
- Small footprint
- Four user-programmable protocols
- Adjustable audio continuity tone indicating active probe Injection counter to indicate total number of injections
NOTE: Microinjection pump and other accessories shown in the system image are sold separately.
Benefits
- Increase viability of injected embryos
- Flexible system
Integrates with WPI’s PV820/PV830, as well as other popular microinjection systems, like the Eppendorf Femtojet® and the Narishige injectors (Femtojet® 4I is a registered Trademark of Eppendorf.)
Microinjection of diverse compounds and biomolecules – DNA, RNA, proteins
Pre- and post-implantation in embryos of various species – mice, rodents, monkeys, bovine, pigs, zebrafish, etc. - Easy to operate – Hands-free operation with foot switch control
- User adjustable frequency and voltage – four programmable protocols
- Small, portable unit saves precious benchtop space
- Works with all major inverted microscopes
Target Applications
- Microinjection into oocytes and pre-implantation stage mammalian embryos, including microinjection of CRISPR-Cas9 reagents into the cytoplasm of two-cell stage embryos
- Pronuclear rodent zygote microinjection
- Gene silencing in zebrafish
Simple, Elegant Solution
The new WPI MICRO-ePORE™ pinpoint cell penetrator is a simple and versatile system that can be used for efficient microinjection of a diverse array of compounds and biomolecules into oocytes and pre-implantation stage mammalian embryos. Patent pending Flutter Electrode Technology assists in small, clean, precise membrane penetration without tearing or damaging the membrane.
Experimental Data
Electrophysiological systems utilizing negative capacitance have been routinely used for microinjection of a variety of biomolecules into mammalian oocytes, as well as pre-implantation and post-implantation embryos in develomental biology studies.1—6 The system which is no longer available, the intracellular amplifier WPI Cyto721, allows the needle to pierce the cell membrane with minimal physical trauma. More recently this technique has been applied to the microinjection of CRISPR/Cas9 reagents into two-cell stage mouse embryos.7 The authors demonstrated significant increase in the knock-in efficiency and high viability of embryos using their method.
The new MICRO-ePORE™ pinpoint cell penetrator offers a unique solution for microinjection resulting in high viability. The instrument creates an oscillating electric field at a localized site on the membrane immediately beneath the site of injection. The MICRO-ePORE™ creates small, reversible holes in the plasma membrane through which material is microinjected. The researcher determines the amplitude and frequency of the signal that best suits the application. In contrast to conventional microinjection, in targeted microinjection using the MICRO-ePORE™, the membrane does not tear and thus allows for superior viability of embryos. The technique is simple and elegant. The new MICRO-ePORE™ cell penetrator prototype has been successfully tested in mouse and primate pre-implantation embryos, as well as gene silencing in zebrafish tails.
MICRO-ePORE™ was designed for a range of applications including generation of CRISPR/Cas9 mediated knock-in mice with large insertions by microinjection into two-cell stage embryos with high viability.7 The MICRO-ePORE™ has delivered accurate microinjection of morpholino oligomers (anti-sense “knockdown”) in zebrafish tails.
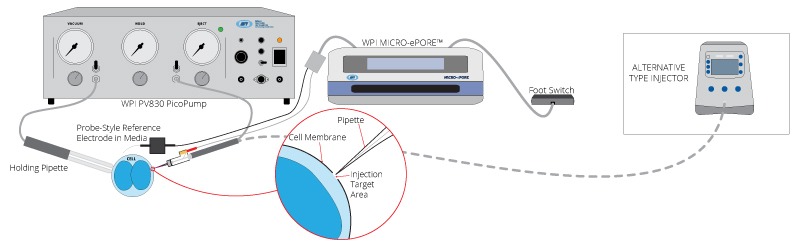
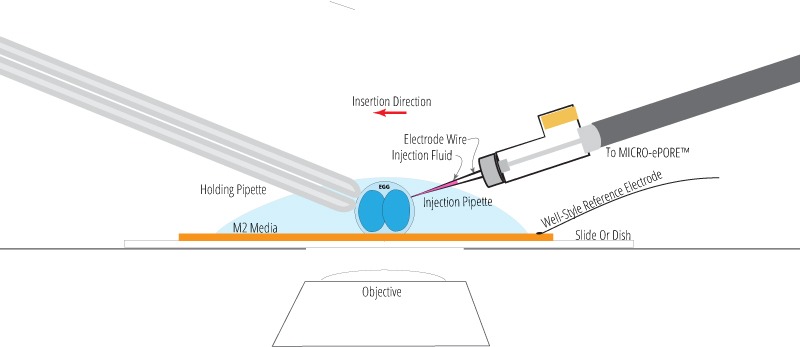
Well-Style Reference Electrode Option
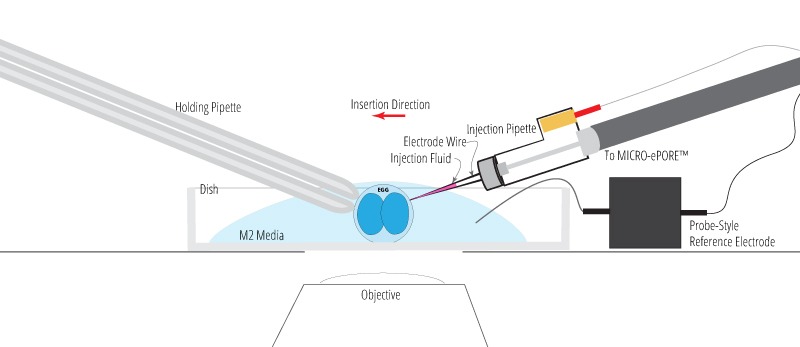
Probe-Style Reference Electrode Option
Unpacking Your MICRO-ePORE™ System
Connecting Your MICRO-ePORE™ System
Connecting the MICRO-ePORE™ Electrode Holders
Specifications
| Voltage parameters | 0–3.0 V, at 1 mV increments |
| Frequency parameters | 50–3000 Hz, at 1 Hz increments |
| Pipette resistance alarm threshold maximum | 500 MΩ |
| Dimensions | 19.7 × 12.7 × 7.6 cm (7.75 × 5 × 3 in.) |
| Weight | 0.9 kg (2 lb.) |
| Certifications | CE, RoHS |
Accessories
Citations
Balakier H, Pedersen RA. Allocation of cells to inner cell mass and trophectoderm lineages in preimplantation mouse embryos. Dev Biol. 1982 Apr; 90(2):352-62. PMID: 7075865 (https://www.ncbi.nlm.nih.gov/pubmed/7075865)
Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987 Nov;101(3):627-52. PMID:3502998 (https://www-ncbi-nlm-nih-gov.myaccess.library.utoronto.ca/pubmed/3502998)
Wianny F, Zernicka-Goetz M. Specific interference with gene function by double-stranded RNA in early mouse development. Nat Cell Biol. 2000 Feb; 2(2):70-5. PMID:10655585 (https://www.ncbi.nlm.nih.gov/pubmed/10655585)
Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006 May; 10(5):615- 24. PMID:16678776 (https://www.ncbi.nlm.nih.gov/pubmed/16678776)
Swann K, Campbell K, Yu Y, Saunders C, Lai FA. Use of luciferase chimaera to monitor PLCzeta expression in mouse eggs. Methods Mol Biol. 2009; 518:17-29. doi: 10.1007/978-1-59745-202-1_2. PMID:19085135 (https://www.ncbi.nlm.nih.gov/pubmed/19085135)
Posfai E, Petropoulos S, de Barros FRO, Schell JP, Jurisica I, Sandberg R, Lanner F, Rossant J. Position- and Hippo signaling-dependent plasticity during lineage segregation in the early mouse embryo. Elife. 2017 Feb 22; 6. pii: e22906. doi: 10.7554/eLife.22906. PMID: 28226240 (https://www.ncbi.nlm.nih.gov/pubmed/28226240)
Gu B, Posfai E, Rossant J. Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nature Biotechnology 2018 Aug; 36(7):632-637. doi: 10.1038/nbt.4166. Epub 2018 Jun 11. PMID: 29889212 (https://www.ncbi.nlm.nih.gov/pubmed/29889212)






Request
Catalogue
Chat
Print