

UPUV-2
UltraPath 2 System for Ultraviolet and Visible Light Absorbance Spectroscopy
- Overview
- Specifications
- Accessories
- Citations
- Related Products
Overview







There are 8 images available to view - click to enlarge and scroll through the product gallery.
Instruction Manual UPUV-2 UltraPath 2 System
/ Download as PDF
UltraPath-2 Data Sheet
/ Download as PDF
- A unique multiple long pathlength sample cell for absorbance spectroscopy
- User-selected optical path lengths: 10, 50 & 200 cm
- Use for Process Control & Oceanography, for very low concentrations and low sample volumes
Details
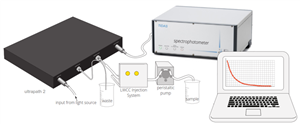
UltraPath™ is a unique high-performance spectrophotometer system offering user-selectable optical path lengths of 10, 50 and 200 cm. The instrument operates in the wavelength range of 250 to 720 nm and has an exceptional dynamic range. Designed for the detection of low absorbing species in aqueous solutions, UltraPath is an ideal tool for any study requiring precise and highly sensitive spectroscopic determination of analytes, either in the lab or in the field.
Background
UltraPath was developed by WPI under a collaborative agreement with NASA (Stennis Space Center) for the spectroscopic determination of colored dissolved organic matter (CDOM) in seawater and fresh water environments. It can be used in the laboratory and in the field (i.e., at sea). CDOM concentrations vary significantly between open ocean samples with low CDOM (e.g., 0.007 m-1 at 380 nm), and high CDOM freshwater environments (e.g., 10-20 m-1 at 380 nm). To address these problems the design requirements of UltraPath mandated the development of a rugged portable system capable of high sensitivity measurements across a wide dynamic range. The UltraPath system meets these stringent design criteria and enables reliable measurement of CDOM in the range of 0.002 m-1 to 230 m-1 (between 250 and 720 nm).
Design
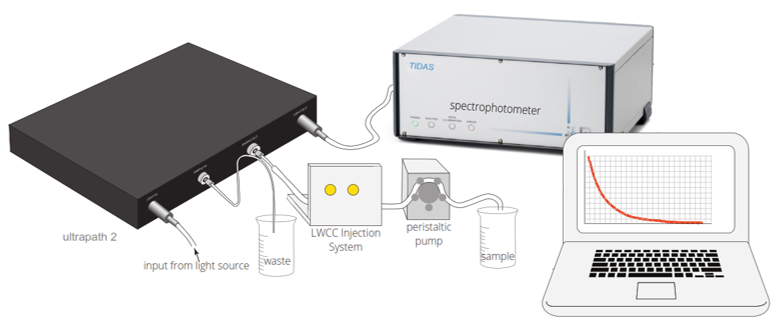
UltraPath has three optical pathlengths contained within a single sample cell (10 cm, 50 cm and 200 cm). The pathlengths are user-selectable, offering a very high sensitivity and an extended dynamic range for UV and VIS absorbance measurements. The fluid path of the sample cell is optimized to produce a laminar flow that is virtually free of interference from trapped air bubbles and adherence of dissolved substances to the cell wall. In particular, the design greatly minimizes the problems commonly found with flow cells of long optical pathlengths: the risk of trapping dust particles, fibers or particulate matter inside the cell. The UltraPath system includes a low noise photodiode array-based spectrometer module.
Mobility
The system is designed for mobility. The components of the UltraPath system are designed to function over a broad range of laboratory and field environments.
Samples
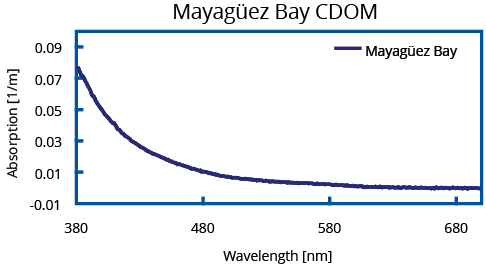
Two typical absorption spectra recorded with an UltraPath of a seawater and a fresh water sample collected in November 2007 are shown below. Due to their high absorbance, both samples were analyzed in the 10 cm pathlength. The CDOM sample labeled Mayagüez Bay in Fig. 2 is from oligotrophic, low productive waters with high salinity collected off the west coast of Puerto Rico in the Mayagüez Bay. Special attention should be drawn to the exceptional sensitivity of UltraPath enabling detection of CDOM absorption below 0.03 m-1. To exemplify the performance of the UltraPath in Laboratory Chemistry and Process Control, Ponceau S absorbance was measured with the 200 cm pathlength of an UltraPath. Normalizing the Ponceau absorbance graph to AU/cm, the range of this measurement is 150 μAU with a noise level below 2 μAU peak to peak. Sub-nanomolar concentration of this dye can clearly and reliably be detected, which is a novelty in absorbance based spectroscopy.
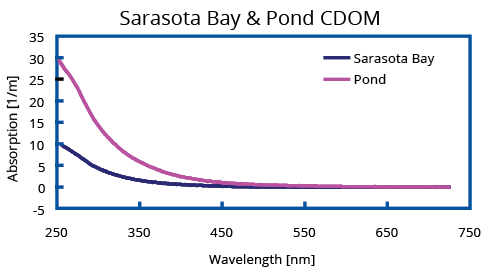
(Left) Two typical absorption spectra measured using UltraPath. The sample labeled “Sarasota Bay” is a CDOM sample with 34 PSU salinity collected from Sarasota Bay (Nov. 2007), and the sample labeled “Pond” is a highly concentrated CDOM sample collected from a local pond in Sarasota, Florida (Nov. 2007).
(Right) CDOM Sample “Mayagüez Bay” was collected from the high salinity oligotrophic waters of Mayagüez Bay on the west coast of Puerto Rico (2001). Data courtesy of NASA Stennis Space Center.
Particulate absorption
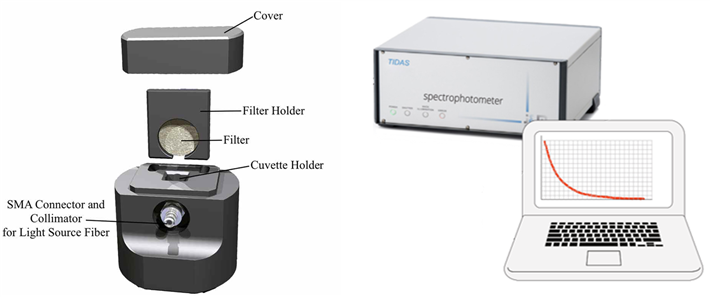
Particulate absorption can be measured by the well established Quantitative Filter Technique (QFT). The UltraPath 2 system includes a fiber optic filter holder for Glass Fiber Filters which can be used with the spectrophotometer so that particulate absorption can be measured on site, avoiding loss of spectral information due to freezing and shipping particulate samples to a laboratory.
Specifications
| Dynamic range |
0.002 m-1 – 230 m-1 (Absorption) |
| Wavelength Range | 250 nm to 720 nm |
| Wavelength Resolution (FWHM) | 5 nm |
| Noise (peak to peak ) | < 0.2 mAU |
| Drift | < 1 mAU/h |
| Optical Pathlength | 10, 50 & 200 cm (user selectable) |
| Sample cell inner diameter | ~1 mm |
| Cell Volume | 2.4 mL (at 200 cm pathlength) |
| Sample inlet / outlet | 1/8 " |
| Fiber Inout/Output | 600 μm, SMA |
| Solvent Resistance | Most organic and inorganic solvents |
| Shipping Weight | 34 kg |
Accessories
Citations
Swan, C., Nelson, N., Siegel, D., & Fields, E. (2013). A model for remote estimation of ultraviolet absorption by chromophoric dissolved organic matter based on the global distribution of spectral slope. Remote Sensing of …. Retrieved from https://www.sciencedirect.com/science/article/pii/S0034425713001624
Matsuoka, A., Babin, M., & Doxaran, D. (2014). A synthesis of light absorption properties of the Arctic Ocean: application to semianalytical estimates of dissolved organic carbon concentrations from space. Biogeosciences. Retrieved from https://hal.upmc.fr/hal-01308049
D’Sa, E., Goes, J., Gomes, H., & Mouw, C. (2014). Absorption and fluorescence properties of chromophoric dissolved organic matter of the eastern Bering Sea in the summer with special reference to the influence of a. Biogeosciences. Retrieved from https://www.biogeosciences.net/11/3225/2014/
Antoine, D., Hooker, S., & Bélanger, S. (2013). Apparent optical properties of the Canadian Beaufort Sea–Part 1: Observational overview and water column relationships. …. Retrieved from https://www.biogeosciences.net/10/4493/2013/bg-10-4493-2013.pdf
Powers, L., & Miller, W. (2016). Apparent quantum efficiency spectra for superoxide photoproduction and its formation of hydrogen peroxide in natural waters. Frontiers in Marine Science. Retrieved from https://journal.frontiersin.org/article/10.3389/fmars.2016.00235/full
Matsuoka, A., & Ortega-Retuerta, E. (2015). Characteristics of colored dissolved organic matter (CDOM) in the Western Arctic Ocean: relationships with microbial activities. Deep Sea Research …. Retrieved from https://www.sciencedirect.com/science/article/pii/S0967064515000417
Andrew, A., & Vecchio, R. Del. (2013). Chromophoric dissolved organic matter (CDOM) in the Equatorial Atlantic Ocean: optical properties and their relation to CDOM structure and source. Marine Chemistry. Retrieved from https://www.sciencedirect.com/science/article/pii/S0304420312001314
Andrew, A. (2014). Chromophoric dissolved organic matter (CDOM) in the ocean: Optical properties and relation to CDOM structure and source. Retrieved from https://drum.lib.umd.edu/handle/1903/15830
Majestic, B. J., Schauer, J. J., Shafer, M. M., Turner, J. R., Fine, P. M., Singh, M., & Sioutas, C. (2006). Development of a wet-chemical method for the speciation of iron in atmospheric aerosols. Environmental Science & Technology, 40(7), 2346–51. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16646472
Zheng, G., Stramski, D., & Reynolds, R. (2014). Evaluation of the Quasi-Analytical Algorithm for estimating the inherent optical properties of seawater from ocean color: Comparison of Arctic and lower-latitude waters. Remote Sensing of Environment. Retrieved from https://www.sciencedirect.com/science/article/pii/S0034425714003174
Miller, R., López, R., & Mulligan, R. (2014). Examining Material Transport in Dynamic Coastal Environments: An Integrated Approach Using Field Data, Remote Sensing and Numerical Modeling. Remote Sensing and …. Retrieved from https://link.springer.com/chapter/10.1007/978-3-319-06326-3_14
Bélanger, S., Cizmeli, S., & Ehn, J. (2013). Light absorption and partitioning in Arctic Ocean surface waters: impact of multiyear ice melting. …. Retrieved from https://search.proquest.com/openview/7e8820a71977904800e9d14b78eb72cc/1?pq-origsite=gscholar&cbl=105740
Bricaud, A., Babin, M., Claustre, H., Ras, J., & Tièche, F. (2010). Light absorption properties and absorption budget of Southeast Pacific waters. Journal of Geophysical Research, 115(C8), 1–19. https://doi.org/10.1029/2009JC005517
Hashihama, F., Kanda, J., Tauchi, A., & Kodama, T. (2015). Liquid waveguide spectrophotometric measurement of nanomolar ammonium in seawater based on the indophenol reaction with o-phenylphenol (OPP). Talanta. Retrieved from https://www.sciencedirect.com/science/article/pii/S0039914015003574
Amornthammarong, N., & Zhang, J.-Z. (2009). Liquid-waveguide spectrophotometric measurement of low silicate in natural waters. Talanta, 79(3), 621–6. https://doi.org/10.1016/j.talanta.2009.04.050
Organelli, E., Bricaud, A., & Gentili, B. (2016). Retrieval of Colored Detrital Matter (CDM) light absorption coefficients in the Mediterranean Sea using field and satellite ocean color radiometry: Evaluation of bio-. Remote Sensing of …. Retrieved from https://www.sciencedirect.com/science/article/pii/S0034425716303315
Organelli, E., & Bricaud, A. (2014). Seasonal dynamics of light absorption by chromophoric dissolved organic matter (CDOM) in the NW Mediterranean Sea (BOUSSOLE site). Deep Sea Research Part I: …. Retrieved from https://www.sciencedirect.com/science/article/pii/S0967063714000764
Wear, E., & Carlson, C. (2015). Synchronous shifts in dissolved organic carbon bioavailability and bacterial community responses over the course of an upwelling-driven phytoplankton bloom. Limnology and …. Retrieved from https://onlinelibrary.wiley.com/doi/10.1002/lno.10042/full
Sempéré, R., & Para, J. (2015). Variability of solar radiation and CDOM in surface coastal waters of the Northwestern Mediterranean Sea. Photochemistry and …. Retrieved from https://onlinelibrary.wiley.com/doi/10.1111/php.12434/full




Request
Catalogue
Chat
Print