In Vitro Evaluation of Endothelial Cell Activation
Optimized, in vitro workflow to identify compounds with vasoactive properties
In Vitro Evaluation of Endothelial Cell Activation Using TEER and Cell Culture Inserts
In vitro models have employed two common methods to quantify changes in endothelial barrier integrity: transepithelial/transendothelial electrical resistance (TEER) and tracer compound permeability.(1) TEER is a non-invasive method that quantifies changes in electrical conductance to measure confluency and barrier integrity. Tracer compound permeability uses molecules of defined molecular weights to measure the size exclusion capacity of cell barriers (e.g., 4 kDa FITC-dextran or FD4).(1) Using the EVOM™ Manual (EVOM-MT-03-01) with the EndOhm TEER electrode and cell culture permeable supports, this application note describes how to non-invasively evaluate endothelial barrier integrity after cytokine treatment and provides a method to identify vasoactive compounds that have the potential to induce vascular injury. Tracer compound permeability studies are combined with TEER evaluation to elucidate treatment-induced impacts on both intercellular junctions and paracellular transport (Fig. 1).
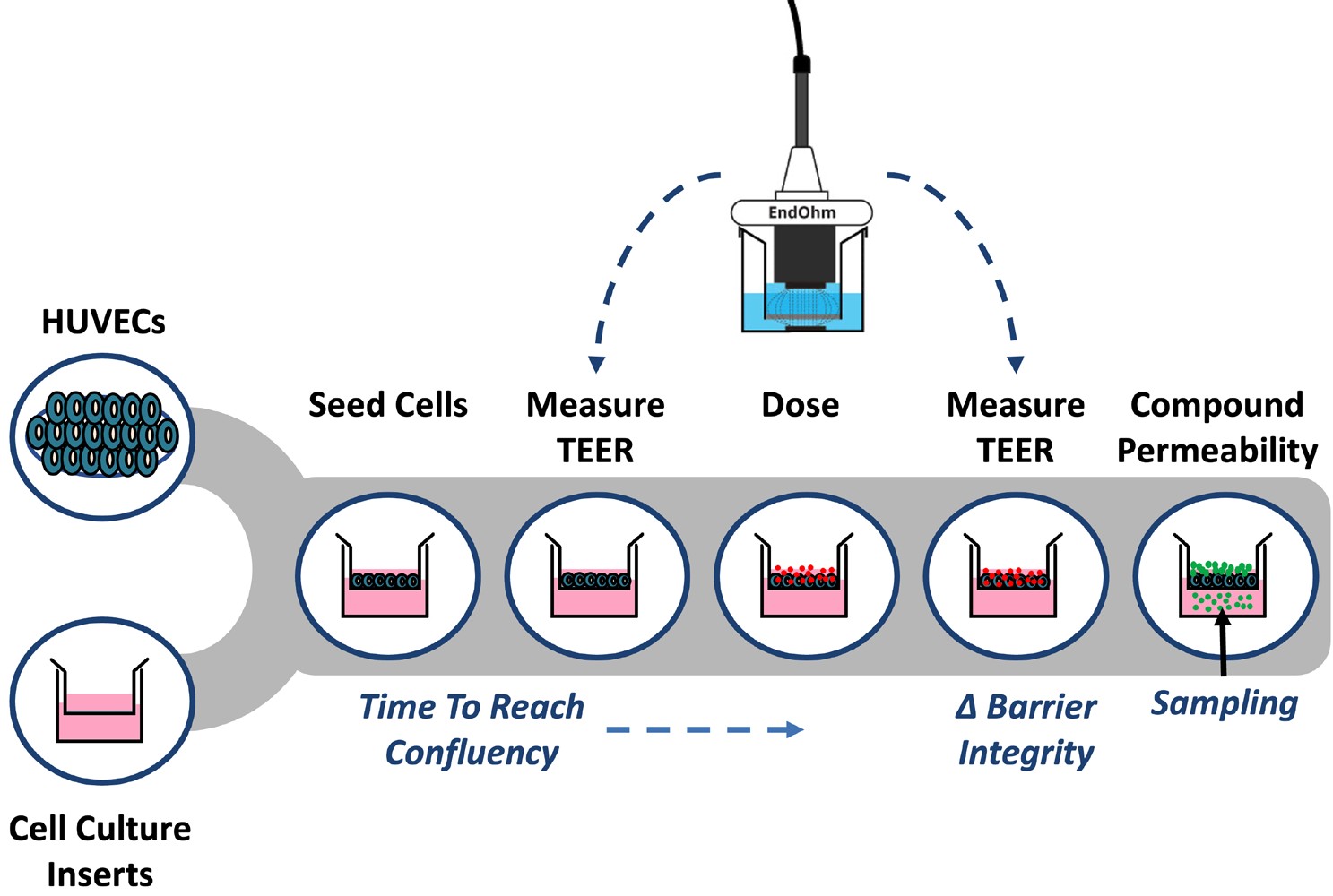
Fig. 1– Workflow for measuring endothelial activation using TEER and tracer permeability assays.
Drug-induced vascular injury (DIVI) occurs when vasoactive compounds damage the endothelial vessel and surrounding smooth muscle, causing inflammation and development of vascular lesions.(2) DIVI is difficult to predict and can be induced by drugs from diverse classifications and disease-specific categories.(3) DIVI-related drug toxicity impacts around 2.5% of new drug candidates, making early identification of vasoactive compounds critical for preclinical drug development.(4) Endothelial cell (EC) activation is an important precursor step in DIVI.(5) ECs adopt this pro-inflammatory phenotype in response to disease (e.g., sepsis, viral hemorrhagic fever), cytokines, external mechanical forces, or drug treatment.(6) EC activation damages and alters the endothelial vessel integrity, leading to changes in vascular permeability, vasomotor tone, and biomarker expression (e.g., E-selectin, P-selectin, ICAM1, VCAM1).(7) Vascular permeability is controlled by a system of intercellular junctions that include tight junctions (TJs) and adherens junctions (AJs).(8) These protein-protein interactions form a selectively permeable barrier that supports paracellular transport and maintains vascular integrity.(9) When endothelial cells are activated in response to treatment with vasoactive compounds, focal endothelial gaps can form due to changes in the organization of tight junction proteins. These gaps alter barrier integrity and pore size of the intercellular junctions, leading to dysregulated endothelial permeability or vascular “leakiness.”(8)
To establish an in vitro model for measuring endothelial cell activation, human umbilical vein endothelial cells (HUVECs) were seeded into cell culture inserts at a density of 6 x 104 cells/cm2 (Fig. 1). TEER was measured using the EVOM™ Manual with the EndOhm TEER electrode over several days until confluency was reached. Confluent HUVECs were treated with either 10 or 50 ng/ml TNF-alpha for 24 hours and measured by TEER. A fluorescent tracer (4 kDa FITC-dextran) was added to the apical chamber for 1 hour, and the amount of tracer in the basolateral chamber was measured using a plate reader (Ex/Em 495/535).
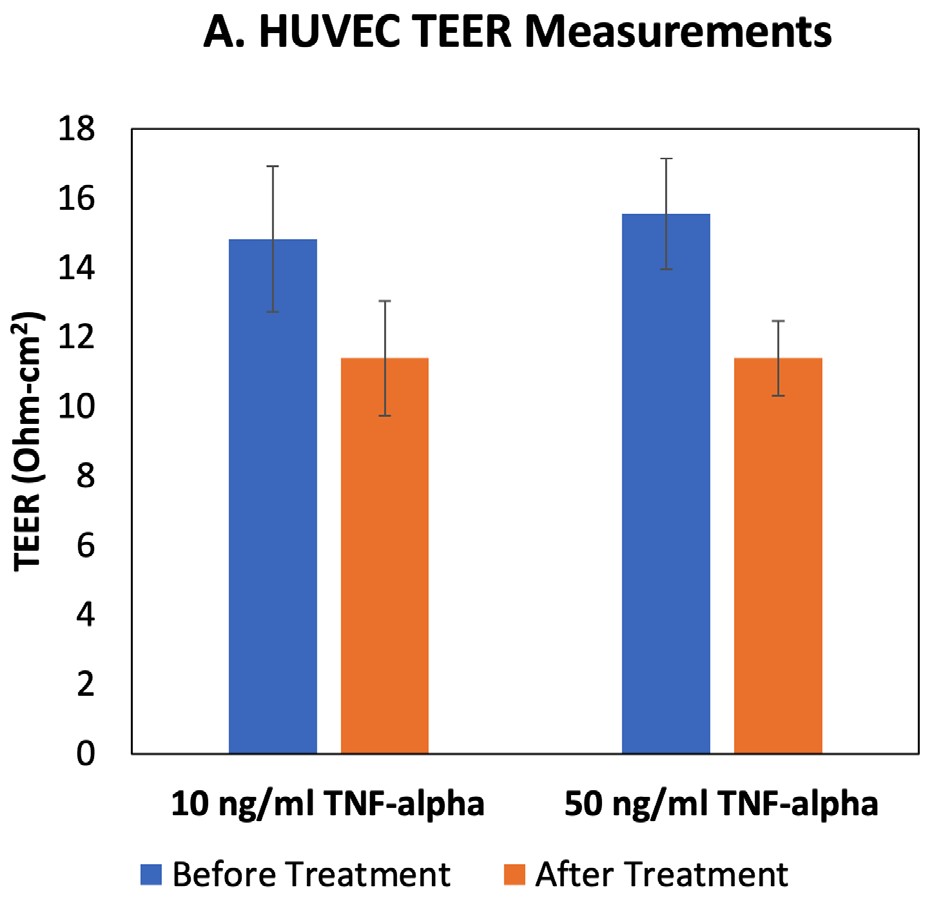
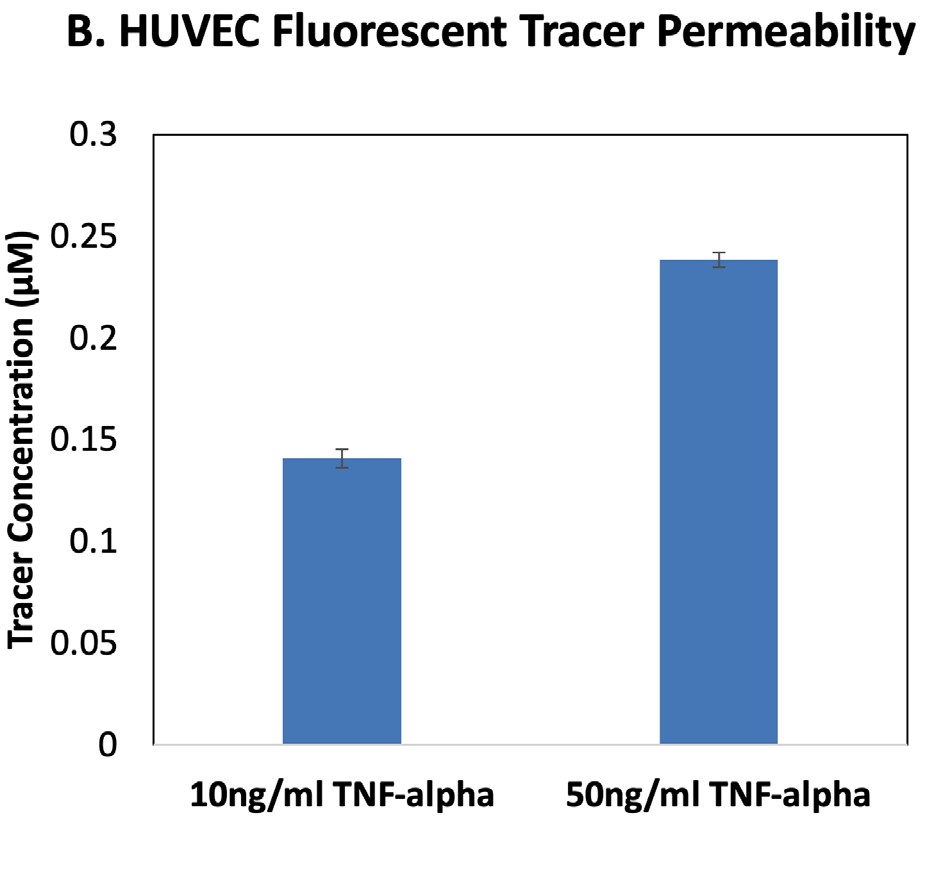
Fig. 2–TEER and fluorescent tracer permeability results for HUVECs treated with TNF-alpha.
The EVOM™ Manual with the EndOhm TEER electrode measured a decrease in HUVEC TEER after 24 hr TNF-alpha treatment, and the lower TEER values were similar for both 10 and 50 ng/ml doses (Fig. 2A). Permeability analysis using a fluorescent tracer showed that HUVECs treated with 50 ng/ml TNF-alpha were more permeable to the fluorescent tracer than the 10 ng/ml-treated
cells at this time point (Fig. 2B). Permeability of paracellular tracers like FITC-dextran depends on the pore size of the tight junctions and paracellular water flow, whereas TEER values are impacted by the ionic conductance.1
HUVECs treated with TNF-alpha show changes in the size exclusion capabilities of the tight junctions (permeability; Fig. 2B), as well as changes in paracellular transport (TEER; Fig. 2A). Together these data demonstrate how the EVOM™ Manual with the EndOhm electrode can be used in combination with permeability tracer assays to evaluate the impacts of cytokines on intercellular junctions and changes in endothelial activation states.
NOTE: If you are interested in contract research services for evaluating drug-induced treatment effects on endothelial barrier integrity, contact SynVivo Inc. at support@synvivobio.com
References
1. Felix K, Tobias S, Jan H, Nicolas S, Michael M. (2021) Histochem Cell Biol. 156(6):609-616. PMID 34459960
2. Yaseen K, Nevares A, and Tamaki H. (2022) Curr Rheumatol Rep. 24(11):323-336. PMID 36129631
3. Zhang J, Hanig JP, and De Felice AF. (2012) Vascul Pharmacol. 56(1-2):14-25. PMID 21968053
4. CDER U.S. Food and Drug Administration. Innovative Medicines Initiative – TransBioLine Drug-induced Vascular Injury Work Package DDTBMQ000037 Letter of Intent. U.S. Food and Drug, Silver Spring, MD, 2019.
5. Ricard N, Bailly S, Guignabert C, and Simons M. (2021) Nat Rev Cardio. 18:565-580. PMID 33627876
6. Siddal E, Khatri M, and Radhakrishnan J. (2017) Kidney Int. 92(1):37-46.
7. Frazier KS, Engelhardt JA, et al. (2015) Toxicol Pathol. 43(7):915-934. PMID 25722122
8. Claesson-Welsh L, Dejana E, and McDonald DM. (2021) Trends Mol Med. 27(4):314-331. PMID 33309601
9. Srinivasan B, Kolli AR, et al. (2015) J Lab Autom. 20(2):107-126. PMID 25586998
EVA-MT-03-01
EVOM Auto For TEER Measurement in 96 HTS Plate
System includes:
- EVOM™ Auto Autosampler
- HTS Electrode Array for 96 HTS Plates
- Interface Unit and Cable
- Control iPad with software
EVA-MT-03-02
EVOM Auto For TEER Measurement in Corning 24 HTS Plates
System includes:
- EVOM™ Auto Autosampler
- HTS Electrode Array for Corning 24 HTS Plates
- Interface Unit and Cable
- Control iPad with software
EVA-MT-03-03
EVOM Auto For TEER Measurement in Millipore 24 HTS Plates
System includes:
- EVOM™ Auto Autosampler
- HTS Electrode Array for Millipore 24 HTS Plates
- Interface Unit and Cable
- Control iPad with software

EVM-EL-03-01-01
ENDOHM-6 EVOM™ Electrode for TEER in 6.5 mm Insert, for use with EVOM Manual a...

EVM-EL-03-01-02
ENDOHM-12 EVOM™ Electrode for TEER in 12 mm Insert, for use with EVOM Manual a...
.png)
EVM-EL-03-01-03
ENDOHM-24 EVOM™ Electrode for TEER in 24 mm Insert, for use with EVOM Manual a...










Request
Catalogue
Chat
Print