
Automation and Optimization Of A 3D Blood Brain Barrier Model
Automation and Optimization of a Three-Dimensional  Blood Brain Barrier Model
Blood Brain Barrier Model
Abstract
Technologies
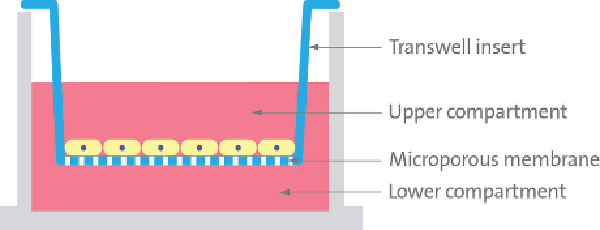
Corning® Transwell® permeable supports allow multiple cell types to be cultured to model the BBB.
Schematic of single insert |
Corning® High Throughput Screening (HTS) Transwell® 96-well Permeable Support |

Materials and Methods
Cell Culture
Primary human Astrocytes (iXCells 10HU-035) were thawed in Dulbecco’s Modified Eagle’s Medium (DMEM; 10-013-CM) supplemented with 10% fetal bovine serum (FBS; 35-010-CV) and maintained on Corning® BioCoat® Collagen I-coated flasks (354486). A working bank of cryopreserved cells were used upon recovery from thaw or after a single passage to a new collagen coated flask. Neuroblastoma cell line SH-SY5Y (ATCC®; CRL-2266) were maintained in DMEM supplemented with 10% FBS. hCMEC/D3 (MilliporeSigma; SCC066) were cultured on Corning® BioCoat® Collagen I-coated flasks in EBM-2 (Lonza; 3156) supplemented with EGM-2 MV (Lonza; 4147), and additional 2.5% FBS, and 1% chemically modified lipid concentrate (Thermo Scientific; 11905031).
24 HTS Set-up
Various combinations of single, co- and tri- cultures were set up using 0.4 μm Corning® HTS Transwell®-24-well Permeable Supports (3379). For inserts cultured with Astrocytes, a 50 μL droplet of 100 μg/mL of Corning® Matrigel® Growth Factor Reduced Basement Membrane Matrix diluted in phosphate buffered saline (PBS; 21-040-CM) was applied to the underside of the insert by inverting plate onto its lid. Inserts were incubated in the laminar flow hood for one hour prior to aspiration followed by seeding of Astrocytes. Astrocytes were harvested with Accutase® Cell Detachment Solution (25-058-CI) and resuspended in DMEM containing 10% FBS at a density of 264,000 cells/mL. A 50 μL droplet of cells (75,000 cells/cm2) was added to each inverted insert and cells were allowed to attach for one hour prior to inverting plate to standard culture position and filling inserts with 200 μL and receiver well with 500 μL of DMEM plus 10% FBS. On the same day, receiver plates (3524) that include SH-SY5Y were seeded with Accutase® harvested cells at 10,000 cells/cm2 in 500 μL of DMEM containing 10% FBS. Astrocyte and SY-SY5Y cultures were incubated separately for approximately 72 hours prior to hCMEC/D3 addition. hCMEC/D3 were harvested with Accutase and seeded into inserts with or without Astrocytes at a density of 40,000 cells/cm2 in 300 μL of hCMEC/D3 assay media. hCMEC/D3 assay media consisted of EBM-2 supplemented with EGM (Lonza; 4133) minus bovine brain extract, an additional 3% FBS, and 1% chemically modified lipid concentrate. Inserts were combined with cell culture plates with or without SH-SY5Y cells using 1 mL of hCMEC/D3 assay media in the cell culture plate below. Media was exchanged every other day. Forty-eight hours after hCMEC/D3 addition, some cultures were exposed to shear conditions by placing on a Corning® LSE™ Low Speed Orbital Shaker (6780-FP) at 50 RPM. TEER was measured daily after addition of hCMEC/D3 cells using World Precision Instruments EVOM™ AUTO (EVA-MT-03-02).
Tight Junction Staining
24 HTS monolayers were fixed by aspirating medium and replacing with 500 μL of 4% paraformaldehyde (PFA; Boston BioProducts; BM-155) per insert and 1mL per cell culture plate. After 15 minutes at room temperature, inserts were washed with the same volume of PBS. Monolayers were then permeabilized with 0.1% Triton X Integra Chemical Company; T756.30.30) for 15 minutes and washed with PBS again. Monolayers were then blocked with 2% bovine serum albumin (MilliporeSigma; A9576) in PBS overnight at 2-8º C. The next day cells were washed with PBS and monolayers were stained with 100 μL of 1:1000 ZO-1 (Invitrogen; 740002MP647) overnight at 2-8º C.
96 HTS Set-up
After optimization in Corning® HTS Transwell® 24-well Permeable Supports, the same protocol was applied to 0.4 μm Corning® HTS Transwell® 96 Permeable Supports (7369) using only Astrocyte with hCMEMC/D3 and SH-SY-5Y with hCMEMC/D3 conditions. Matrigel coating and Astrocyte seeding volumes to the underside of the inserts were decreased to 22 μL per insert and the concentrations were adjusted to maintain the same μg or cells per cm2. After Astrocyte cell attachment, 100 μL of DMEM with 10% FBS was added to each insert and 200 μL per receiver well. Additionally, SH-SY-5Y cells were seeded into Corning HTS Transwell-96 Receiver Plates (3382) in 200 μL per well. Shear was applied to all inserts after 48 hours of hCMEC/D3 attachment. TEER was measured daily using the EVA-MT-03-01 electrode array.
Compound Addition
Approximately, 120 hours after hCMEC/D3 addition, media was aspirated from Corning® HTS Transwell® 96 Permeable Supports and replaced with serially diluted Lipopolysaccharide (LPS; Invitrogen; 00-4976-93), Insulin (Thermo Scientific; 12585014), TNF alpha (Thermo Scientific; 300-01A-500 μg) or media as a negative control. Compounds were incubated with cultures on shaker for 48 hours with TEER being measured daily.
In Vitro Establishment of BBB and Evaluation of Compound Effect on Permeability
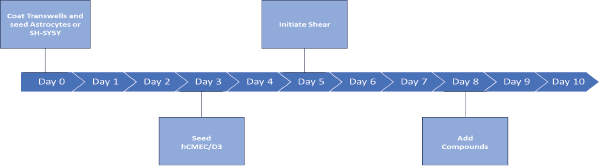
Schematic of a 10-day workflow for assay.
Results
24 HTS Optimization

Immunocytochemistry Staining
TEER is a non-destructive functional readout of barrier integrity analytical technique which can be used to monitor changes to 2D and 3D tissues models and correlate in vitro and in vivo phenomena.
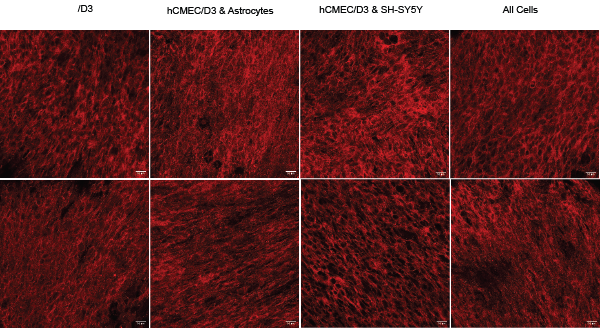
Figure 2: ZO-1 Immunohostochemistry shows increased tight junctions and BBB integrity of hCMEC/D3 cells under shear stress. Representative images of various cell combinations on permeable supports cultured under static (top row) or shear conditions (bottom row). Cultures fixed on day 8. Images taken with Leica Stellaris 5 laser scanning confocal with a 40x/1.3 NA oil immersion objective lens. Scale is 20 μm.
96 HTS TEER
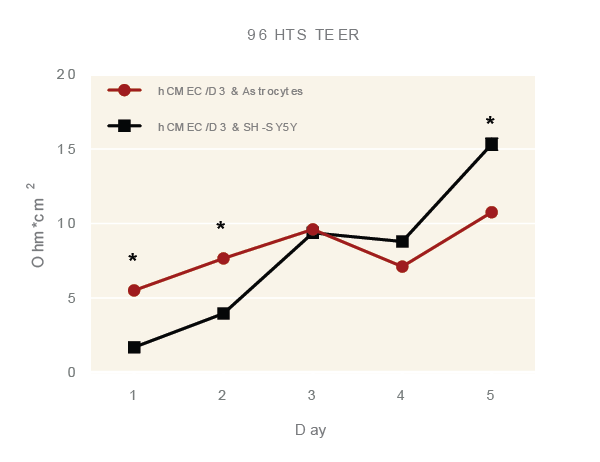
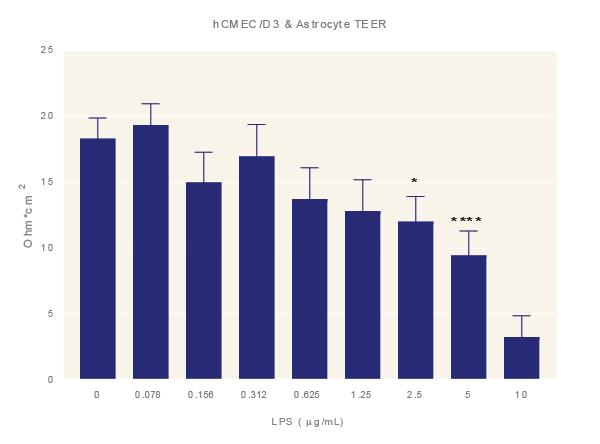 | 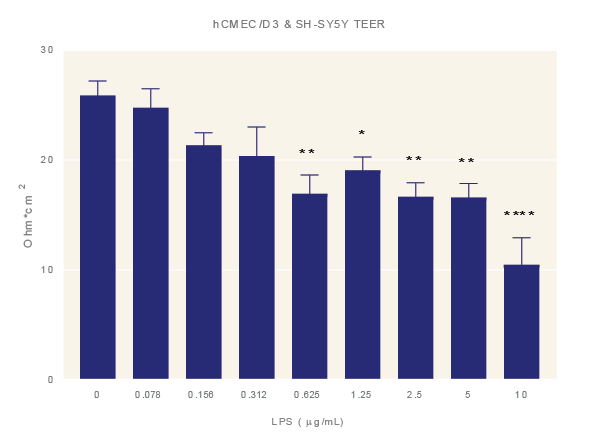 |
Figure 4: LPS increases BBB permeability. Impact of LPS. Average TEER measurement of cultures exposure to increasing concentrations of LPS for 48 hours. Data is the average of three technical replicates per experiment and three independent experiments shown with standard error. ANOVA with Dunnett’s post test in comparison to media only. *=p<0.05, **=p<0.005, ***=p<0.001 ****=p<0.0001.
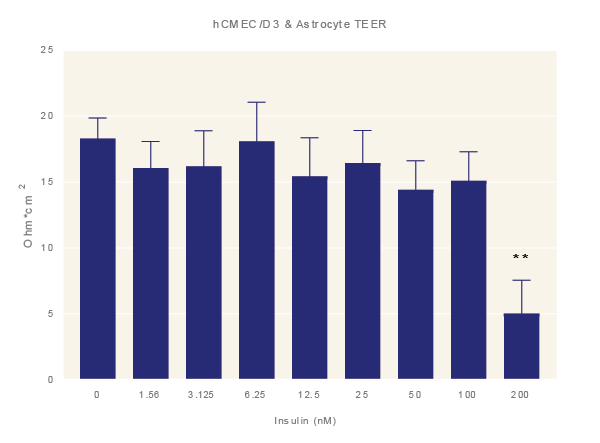 |  |
Impact of Insulin. Average TEER measurement of cultures exposure to increasing concentrations of insulin for 48 hours. Data is the average of three technical replicates per experiment and three shown with standard error. ANOVA with Dunnett’s post test in comparison to media only. *=p<0.05, **=p<0.005, ***=p<0.001 ****=p<0.0001.
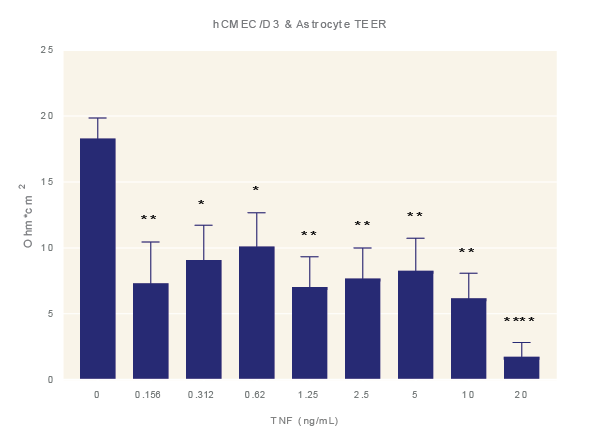 | 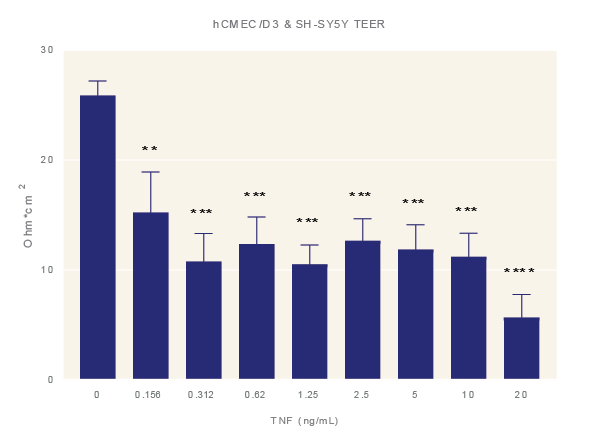 |
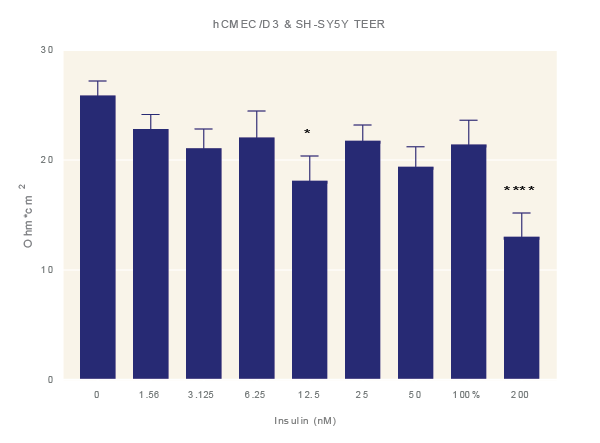
Impact of TNF-α. Average TEER measurement of cultures exposure to increasing concentrations of TNF-α for 48 hours. Data is the average of three technical replicates per experiment and three shown with standard error. ANOVA with Dunnett’s post test in comparison to media only. *=p<0.05, **=p<0.005, ***=p<0.001 ****=p<0.0001.
Conclusions
Adding shear via a low-speed orbital shaker and/or additional BBB relevant cell types such as primary astrocytes or SH-SY5Y can statistically increase TEER readings compared to static culture hCMEC/D3 barriers.
Immunocytochemistry staining shows the appearance of more linear alignment of hCMEC/D3 cells grown under shear conditions.
The EVOM™ Auto rapidly and reproducibly automates measurements of TEER in endothelial monolayers cultured on Corning Transwell 24-well and 96-well Permeable Supports. The ability to monitor barrier integrity aseptically and non-destructively enables researchers to efficiently implement TEER as a real-time measurement of permeability for long-term, longitudinal studies, with reduced cost and variability.
The combination of the EVOM Auto and Corning Transwell permeable supports allow for easy barrier model optimization and higher throughput assays.





Request
Catalogue
Chat
Print