

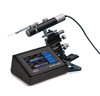
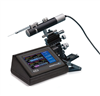
Microinjection Syringe Pump/SMARTouch Controller
Delivering picoliter volumes precisely
- Graphic display with SMARTouch™ touch screen controller for "intelligent", easy to use interface controlling up to four microliter syringe pumps
- Splash proof touch screen
- User configurable mounting bar
- Dual mode motor drive
- Compatible with all UMP, UMP2 and UMP3 micro syringe pumps
- Optional foot switch available
- 5 digit display
Options
| Order code | Description |
| UMP3 | UltraMicroPump3 ONLY (without controller) |
| UMP3T-1 | UltraMicroPump3 (one) and SMARTouch Controller |
| UMP3T-2 | UltraMicroPump3 (two) and SMARTouch Controller |
| MICRO2T | SMARTouch Controller ONLY, Two-Channel |
Note on Accessory
*13142 is the foot pedal for use with the MICRO2T/MICRO4T ONLY. It is not compatible with the old MICRO4 controller, which uses the 15867 foot pedal. The two foot pedals are not cross-compatible.
Benefits
- Accepts a wide range of microinjection syringes from 0.5 ul to 1000 ul.
- Manual or automated injections
- Quiet operation for electrophysiology recordings
- Mounts directly on micromanipulator or stereotaxic frame
- Nominal injections down to 1 nL
- Rapid setup with intuitive touchscreen controller
Applications
- Microinjection
- Neuroscience
- Microfluidics
- Micro delivery of biochemical agents or dyes
- Intravitreal Injection (with RPE-KIT)
- Intraoccular Injection (with IO-KIT)
The UltraMicroPump3 (UMP3) is a versatile microinjection syringe pump which uses micro syringes to deliver picoliter to milliliter volumes. The microinjection syringe pump is optimum for applications that require injections of precise and small amounts of liquid. With its touchscreen controller, UMP3 microinjection syringe pump can displace as little as 0.53 µL/step (using 10 µL syringe with 60 mm scale length).
Patent Pending technology
The new SMARTouch™ controller for the UltraMicroPump3 features Patent Pending technology which includes:
- Total system calibration – Calibrate the syringe and the controller together as a system. This feature eliminates the variability of the syringes and delivers the calibrated volume.
- Smart smoothness – The controller can be set to automatically adjust microstepping according to the injection rate to deliver the smoothest flow.
- User defined travel limits – Set the limits for a specific syringe in the software. This prevents the micro syringe pump from over-driving the plunger into the syringe, potentially causing syringe breakage.
The MICRO2T SMARTouch™ controller is feature rich. All operations are controlled through interactive touch screen. It has a graphical indication of the flow and the volume remaining in the syringe. It offers end stop detection that is dependent on the syringe volume. You can control two pumps independently from one controller with its dual display. It also has automatic pump detection and a Pause/Resume feature that allows dosing during infusion/withdrawal. The volume accumulated is displayed on screen, as well as the percentage of volume left in the syringe. The SMARTouch™ controller is fully compatible with all earlier versions of the UltraMicroPump.
Low Fluid Dead Volume
Syringes may be filled externally and then inserted into the pump or filled while mounted in the micro syringe pump. Fluids injected or withdrawn are held entirely within the micro syringe to maintain a low fluid dead volume.
Flexibility in Mounting
For positioning, the UMP3 microinjection syring pump may be attached to any of several WPI micropositioners such as the M3301 (manual), DC3001 (motorized) or any manual stereotaxic manipulator.
Rapid Setup with Intuitive Touchscreen Controller
An Integral component in the UMP3 system is the SMARTouch™ touchscreen controller, which provides an “intelligent” and easy-to-use interface up to two syringe pumps. Operating parameters are set with the touchscreen panel. An optional footswitch offers “hands free” start/stop operation.
Computer Control—A USB port on the rear of the controller can be used to connect it to a computer for scripted protocols.
NOTE: UMP3 accepts glass syringes with barrel diameters from 5.5 to 9 mm.
Application Notes
The following images show various setups for microinjection. Keep in mind that parts are interchangeable.
UMP3T-2

The SMARTouch™ controller can control two UMP3 microliter syringe pumps or Nanoliter Injector pumps simultaneously, either grouped together or independently.
RPE Kit in a custom holder

The RPE Kit is designed for retinal pigment epithelium injections in the eye, using a NanoFIl syringe mounted on the UMP3 microinjection pump. You get accurate, repeatable, oil free injections down to the submicroliter range.
UMP3 with Stereotaxic Frame

The UMP3 microinjection syringe pump is designed to be mounted on any standard stereotaxic frame. Here the microinjection pump is mounted on our motorized stereotaxic frame.
Ultramicropump specifications
(based on 10 μL syringe)
| Normal Mode | |
| Travel | 62 mm |
| Minimum dispensing volume | 0.58 nL / step (10 µL syringe) |
| Linear motion per step | 3.175 µm/half step |
| Weight | 325 g (11.5 oz) |
| Mounting rod diameters | 7.9 mm (0.31 in.) |
| Mains power supply | 90-264VAC @ 47-63Hz |
| Dimensions | ∅ 32 mm x 190 mm (∅ 1.3 in. x 7.5 in.) |
| Microstepping Mode | |
| Precision is increased eight-fold. |
UMP3 Pump now compatible with Hamilton Neuro Series7000 Syringe
Video demonstrating how to add NEW plunger button part number 65259 (Call for pricing)
References
Zhou, Z., Luther, N., Singh, R., Boockvar, J. A., Souweidane, M. M., & Greenfield, J. P. (2017). Glioblastoma spheroids produce infiltrative gliomas in the rat brainstem. Child’s Nervous System, 1–10. http://doi.org/10.1007/s00381-017-3344-y
Ye, H.-L., Li, D.-R., Yang, J.-S., Chen, D.-F., De Vos, S., Vuylsteke, M., … Yang, W.-J. (2017). Molecular characterization and functional analyses of a diapause hormone receptor-like gene in parthenogenetic Artemia. Peptides. http://doi.org/10.1016/j.peptides.2017.01.008
Wofford, K. L., Harris, J. P., Browne, K. D., Brown, D. P., Grovola, M. R., Mietus, C. J., … Cullen, D. K. (2017). Rapid neuroinflammatory response localized to injured neurons after diffuse traumatic brain injury in swine. Experimental Neurology, 290, 85–94. http://doi.org/10.1016/j.expneurol.2017.01.004
Qi, Y., Purtell, L., Fu, M., Zhang, L., Zolotukhin, S., Campbell, L., & Herzog, H. (2017). Hypothalamus specific re-introduction of Snord116 into otherwise Snord116 deficient mice increased energy expenditure. Journal of Neuroendocrinology. http://doi.org/10.1111/jne.12457
Mosberger, A. C., Miehlbradt, J. C., Bjelopoljak, N., Schneider, M. P., Wahl, A.-S., Ineichen, B. V., … Schwab, M. E. (2017). Axotomized Corticospinal Neurons Increase Supra-Lesional Innervation and Remain Crucial for Skilled Reaching after Bilateral Pyramidotomy. Cerebral Cortex, 137, 1716–1732. http://doi.org/10.1093/cercor/bhw405
Job, M. O., & Kuhar, M. J. (2017). CART peptide in the nucleus accumbens regulates psychostimulants: Correlations between psychostimulant and CART peptide effects. Neuroscience, 348, 135–142. http://doi.org/10.1016/j.neuroscience.2017.02.012
Eleftheriadou, I., Dieringer, M., Poh, X. Y., Sanchez-Garrido, J., Gao, Y., Sgourou, A., … Mazarakis, N. D. (2017). Selective transduction of astrocytic and neuronal CNS subpopulations by lentiviral vectors pseudotyped with Chikungunya virus envelope. Biomaterials, 123, 1–14. http://doi.org/10.1016/j.biomaterials.2017.01.023
Augestad, I. L., Nyman, A. K. G., Costa, A. I., Barnett, S. C., Sandvig, A., Håberg, A. K., & Sandvig, I. (2017). Effects of Neural Stem Cell and Olfactory Ensheathing Cell Co-transplants on Tissue Remodelling After Transient Focal Cerebral Ischemia in the Adult Rat. Neurochemical Research, 1–11. http://doi.org/10.1007/s11064-016-2098-3
Lin, P., Fang, Z., Liu, J., & Lee, J. H. (2016). Optogenetic Functional MRI. Journal of Visualized Experiments, (110), e53346–e53346. http://doi.org/10.3791/53346
Vacca, O., El Mathari, B., Darche, M., Sahel, J.-A., Rendon, A., & Dalkara, D. (2015). Using Adeno-associated Virus as a Tool to Study Retinal Barriers in Disease. Journal of Visualized Experiments, (98), e52451–e52451. http://doi.org/10.3791/52451
Lai, J., Legault, M.-A., Thomas, S., & Casanova, C. (2015). Simultaneous Electrophysiological Recording and Micro-injections of Inhibitory Agents in the Rodent Brain. Journal of Visualized Experiments, (101), e52271–e52271. http://doi.org/10.3791/52271
Robinson, S., & Adelman, J. S. (2015). A Method for Remotely Silencing Neural Activity in Rodents During Discrete Phases of Learning. Journal of Visualized Experiments, (100), e52859–e52859. http://doi.org/10.3791/52859
Platt, R. J., Chen, S., Zhou, Y., Yim, M. J., Swiech, L., Kempton, H. R., … Zhang, F. (2014). CRISPR-Cas9 Knockin Mice for Genome Editing and Cancer Modeling. Cell, 159(2), 440–55. http://doi.org/10.1016/j.cell.2014.09.014
Pierce, A. M., & Keating, A. K. (2014). Creating Anatomically Accurate and Reproducible Intracranial Xenografts of Human Brain Tumors. Journal of Visualized Experiments, (91), e52017–e52017. http://doi.org/10.3791/52017
Paveliev, M., Kislin, M., Molotkov, D., Yuryev, M., Rauvala, H., & Khiroug, L. (2014). Acute Brain Trauma in Mice Followed By Longitudinal Two-photon Imaging. Journal of Visualized Experiments?: JoVE, (April), 1–8. http://doi.org/10.3791/51559
Nakamura, S., Baratta, M. V., & Cooper, D. C. (2013). A Method for High Fidelity Optogenetic Control of Individual Pyramidal Neurons In vivo Journal of Visualized Experiments, (79), e50291–e50291. http://doi.org/10.3791/50291
Inquimbert, P., Moll, M., Kohno, T., & Scholz, J. (2013). Stereotaxic Injection of a Viral Vector for Conditional Gene Manipulation in the Mouse Spinal Cord. Journal of Visualized Experiments, (73), e50313–e50313. http://doi.org/10.3791/50313
Hewing, N. J., Weskamp, G., Vermaat, J., Farage, E., Glomski, K., Swendeman, S., … Blobel, C. P. (2013). Intravitreal injection of TIMP3 or the EGFR inhibitor erlotinib offers protection from oxygen-induced retinopathy in mice. Investigative Ophthalmology & Visual Science, 54(1), 864–70. http://doi.org/10.1167/iovs.12-10954
Salt, A. N., Hartsock, J. J., Gill, R. M., Piu, F., & Plontke, S. K. (2012). Perilymph Pharmacokinetics of Markers and Dexamethasone Applied and Sampled at the Lateral Semi-Circular Canal. Journal of the Association for Research in Otolaryngology, 13(6), 771–783. http://doi.org/10.1007/s10162-012-0347-y
Nickerson, J. M., Goodman, P., Chrenek, M. A., Bernal, C. J., Berglin, L., Redmond, T. M., & Boatright, J. H. (2012). Subretinal delivery and electroporation in pigmented and nonpigmented adult mouse eyes. Methods in Molecular Biology (Clifton, N.J.), 884, 53–69. http://doi.org/10.1007/978-1-61779-848-1_4
Beier, K., & Cepko, C. (2012). Viral Tracing of Genetically Defined Neural Circuitry. Journal of Visualized Experiments, (68), e4253–e4253. http://doi.org/10.3791/4253
Goel, M., Sienkiewicz, A. E., Picciani, R., Wang, J., Lee, R. K., & Bhattacharya, S. K. (2012). Cochlin, intraocular pressure regulation and mechanosensing. PloS One, 7(4), e34309. http://doi.org/10.1371/journal.pone.0034309
Abdelwahab, M. G., Sankar, T., Preul, M. C., & Scheck, A. C. (2011). Intracranial Implantation with Subsequent 3D In Vivo Bioluminescent Imaging of Murine Gliomas. Journal of Visualized Experiments, (57), e3403–e3403. http://doi.org/10.3791/3403
Lowery, R. L., & Majewska, A. K. (2010). Intracranial Injection of Adeno-associated Viral Vectors. Journal of Visualized Experiments, (45), e2140–e2140. http://doi.org/10.3791/2140
Kinkel, M. D., Eames, S. C., Philipson, L. H., & Prince, V. E. (2010). Intraperitoneal injection into adult zebrafish. Journal of Visualized Experiments?: JoVE, (42), e2126. http://doi.org/10.3791/2126
Molotkov, D. A., Yukin, A. Y., Afzalov, R. A., & Khiroug, L. S. (2010). Gene Delivery to Postnatal Rat Brain by Non-ventricular Plasmid Injection and Electroporation. Journal of Visualized Experiments, (43), e2244–e2244. http://doi.org/10.3791/2244
Marker, D. F., Tremblay, M.-E., Lu, S.-M., Majewska, A. K., & Gelbard, H. A. (2010). A Thin-skull Window Technique for Chronic Two-photon In vivo Imaging of Murine Microglia in Models of Neuroinflammation. Journal of Visualized Experiments, (43), e2059–e2059. http://doi.org/10.3791/2059
Eames, S. C., Philipson, L. H., Prince, V. E., & Kinkel, M. D. (2010). Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish, 7(2), 205–13. http://doi.org/10.1089/zeb.2009.0640
Jasnow, A. M., Rainnie, D. G., Maguschak, K. A., Chhatwal, J. P., & Ressler, K. J. (2009). Construction of Cell-Type Specific Promoter Lentiviruses for Optically Guiding Electrophysiological Recordings and for Targeted Gene Delivery (pp. 199–213). http://doi.org/10.1007/978-1-59745-559-6_13
Christiana J. Johnson, Lennart Berglin, Micah A. Chrenek, T.M. Redmond, Jeffrey H. Boatright, J. M. N. (2008). Technical Brief: Subretinal injection and electroporation into adult mouse eyes. Molecular Vission, 14, 2211–2226. Retrieved from http://www.molvis.org/molvis/v14/a259/
Takayama, K., Torashima, T., Horiuchi, H., & Hirai, H. (2008). Purkinje-cell-preferential transduction by lentiviral vectors with the murine stem cell virus promoter. Neuroscience Letters (Vol. 443).
Torashima, T., Yamada, N., Itoh, M., Yamamoto, A., & Hirai, H. (2006). Exposure of lentiviral vectors to subneutral pH shifts the tropism from Purkinje cell to Bergmann glia. European Journal of Neuroscience, 24(2), 371–380. http://doi.org/10.1111/j.1460-9568.2006.04927.x
Torashima, T., Okoyama, S., Nishizaki, T., & Hirai, H. (2006). In vivo transduction of murine cerebellar Purkinje cells by HIV-derived lentiviral vectors. Brain Research, 1082(1), 11–22. http://doi.org/10.1016/j.brainres.2006.01.104
Dancause, N., Barbay, S., Frost, S. B., Plautz, E. J., Chen, D., Zoubina, E. V, … Nudo, R. J. (n.d.) (2005). Development/Plasticity/Repair Extensive Cortical Rewiring after Brain Injury. https://doi.org/10.1523/JNEUROSCI.3256-05.2005
Cherezov, V., Peddi, A., Muthusubramaniam, L., Zheng, Y. F., & Caffrey, M. (2004). A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases. Acta Crystallographica Section D Biological Crystallography, 60(10), 1795–1807. http://doi.org/10.1107/S0907444904019109
Bernd, A. S., Aihara, M., Lindsey, J. D., & Weinreb, R. N. (2004). Influence of Molecular Weight on Intracameral Dextran Movement to the Posterior Segment of the Mouse Eye. Investigative Opthalmology & Visual Science, 45(2), 480. http://doi.org/10.1167/iovs.03-0462
Shawgo, R. S. (2004). In vivo activation and biocompatibility of a MEMS microreservoir drug delivery device. Retrieved November 16, 2016, from http://citeweb.info/20041104095
Nelson, B. P., Grimsrud, T. E., Liles, M. R., Goodman, R. M., & Corn, R. M. (n.d.) (2001). Surface Plasmon Resonance Imaging Measurements of DNA and RNA Hybridization Adsorption onto DNA Microarrays. https://doi.org/10.1021/ac0010431
Sturbaum, G. D., Reed, C., Hoover, P. J., Jost, B. H., Marshall, M. M., & Sterling, C. R. (2001). Species-Specific, Nested PCR-Restriction Fragment Length Polymorphism Detection of Single Cryptosporidium parvum Oocysts. APPLIED AND ENVIRONMENTAL MICROBIOLOGY, 67(6), 2665–2668. https://www.ncbi.nlm.nih.gov/pubmed/11375178
MICRO2T
SMARTouch Controller ONLY, Two-Channel
- Graphic display with SMARTouch™ touch screen controller for "intelligent", easy to use interface controlling up to four microliter syringe pumps
- Splash proof touch screen
- User configurable mounting bar
- Dual mode motor drive
- Compatible with all UMP, UMP2 and UMP3 micro syringe pumps
- Optional foot switch available
- 5 digit display






Request
Catalogue
Chat
Print