
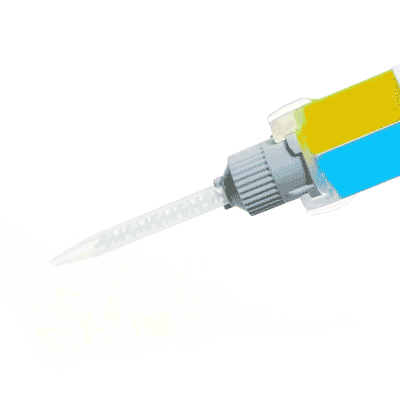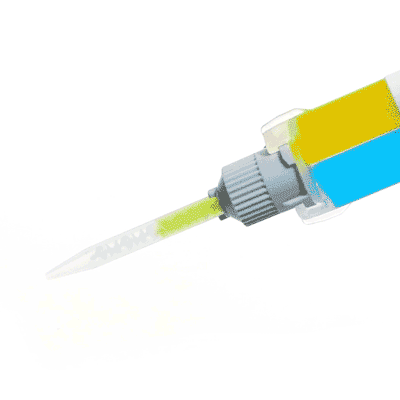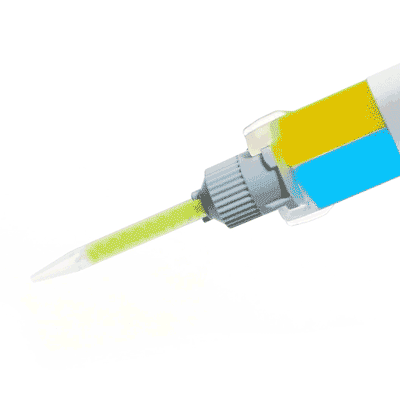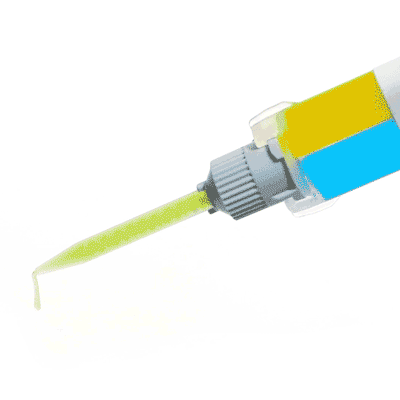
KWIK-SIL
Kwik-Sil™ low toxicity silicone adhesive for live tissues.
Includes 2 x 5 ml cartridges of 2-part silicone and 10 mixing tips.
Kwik-Sil™ is a translucent, medium-viscosity silicone adhesive, developed for chronic peripheral nerve studies such as anterograde tracing with fluorescent indicators or electrode recording. Good adhesion and mechanical properties (tear strength and elongation) allow days of study without breaking of the bonding. Curing speed is very reproducible.
- Overview
- Specifications
- Accessories
- Citations
- Related Products
Overview

There are 5 images available to view - click to enlarge and scroll through the product gallery.
KWIK-SIL / KWIK-CAST Instruction Manual
/ Download as PDF
KWIK-SIL / KWIK-CAST Sales Sheet
/ Download as PDF
Ideal for neuroscience applications, nerve studies and more
- Bio-compatible surgical adhesive for live tissue and nerve studies
- Pre-mixing tips simplify use (Includes 10 mixing tips)
- Medium strength adhesion
- Low toxicity
- Rapid curing silicone adhesive, cure on contact
- Cures without producing heat
- The kit comes with (2) cartridges of 2 part silicone
- Kwik-Sil surgical glue dries clear; Kwik-Cast dries green
- Kwik-Sil surgical glue dries a little faster than Kwik-Cast
KWIK-SIL Overview
The properties of this bio-compatible surgical adhesive make it exceptionally useful for neuroscience applications, peripheral nerve studies and similar biomedical applications. These silicone elastomers also eliminate the mess and time involved in pre-mixing other commonly used formulations (such as Wacker SilGel® and Sylgard®). Each silicone elastomer is packaged in a double barrel syringe and is automatically mixed when pressed out of the mixer tip provided. It can then be applied directly to the tissue without further mixing. The curing time of these silicone adhesives is short, reliable and reproducible. This eliminates potential costly guesswork when using other tissue adhesives. The curing process does not produce any heat, which can cause tissue damage. WPI's silicone elastomers are much less toxic than dental silicone, because they contain no surfactant additives.
The silicone adhesive inside the syringes cannot be sterilized by any means including UV, gamma, H2O2, autoclaving or EtO.
The exterior and the tips can be immersed in or wiped with 70/30 alcohol or you can use a liquid sterilant like Cidex OPA or Rapicide OPA.
Toxicity
WPI's silicone elastomers are based on recently developed vinyl terminated siloxane and platinum complex catalysts. They exhibit exceptional low toxicity before, during and after curing. The traditional RTV silicone systems produce either acetic acid or alcohol during condensation; these compounds are toxic to living cells. In contrast, the only by-product of condensation from WPI™s elastomers is a small amount of hydrogen gas, which was shown not to cause any effect on nerve activity in highly sensitive peripheral nerves. Both elastomers cure on contact with the tissue. Currently, WPI provides rapid-curing silicone elastomers in two different formulas:Kwik-Sil is a translucent silicone elastomer adhesive with medium viscosity. It has good adhesion and mechanical property (tear strength and elongation). The very short curing time (approx. 1 minute) make it especially useful for moving preparations.
Low Toxicity Silicone Adhesive
See for yourself why KWIK-SIL silicone adhesive is so popular for life science applications. When you are looking for a low toxicity adhesive with some elasticity and good moisture resistance, a silicone adhesive is the option of choice. When used with living tissue, an adhesive must be:
- Non-toxic
- Fast Curing
- Able to adhere to organic and inorganic surfaces

Kwik-Sil™ and Kwik-Cast™ silicone adhesives have very low toxicity before, during and after curing. The by-product of curing is a small amount of hydrogen gas, which is much less toxic to cells than acetic acid or alcohol from traditional RTV silicones.
Kwik-Sil™ and Kwik-Cast™ have a curing speed that is hundreds of times faster than traditional RTV silicones. A curing time of a few minutes at room temperature is especially useful for encapsulation of live tissue or implanting into a live animal.
Kwik-Sil™ and Kwik-Cast™ surgical adhesives are not sensitive to contamination from animal tissue, unlike many vinyl-based silicones in which the platinum complex catalysts which are easily poisoned by contamination from amines and animal tissue.
Curing Time
The properties of this bio-compatible surgical adhesive make it exceptionally useful for neuroscience applications, peripheral nerve studies and similar biomedical applications. These silicone elastomers also eliminate the mess and time involved in pre-mixing other commonly used formulations (such as Wacker SilGel® and Sylgard®). Each silicone elastomer is packaged in a double barrel syringe and is automatically mixed when pressed out of the mixer tip provided. It can then be applied directly to the tissue without further mixing. The curing time of these silicone adhesives is short, reliable and reproducible. This eliminating potential costly guesswork when using other tissue adhesives. The curing process does not produce any heat, which can cause tissue damage. WPI's silicone elastomers are much less toxic than dental silicone, because they contain no surfactant additives.
The silicone adhesive inside the syringes cannot be sterilized by any means including UV, gamma, H2O2, autoclaving or EtO.
The exterior and the tips can be immersed in or wiped with 70/30 alcohol or you can use a liquid sterilant like Cidex OPA (WPI P/N 7364-4) or Rapicide OPA (WPI P/N 504611).
Q&A
Q: I want to use KwikSil surgical glue in my "imaging" experiments in rats. How do I get rid of the bubbles completely, or at least reduce them as much as possible?
A: The bubbles are hydrogen gas that is released as a by-product of the curing process. The cure happens in such a short period that the gas can't escape. A slower cure time might help. To slow the cure process, cool the material before mixing.
Kwik-Sil™ New Tip Design

Kwik-Sil™ and Kwik-Cast™ now come with a new tip design. The new tip is engineered to minimize leakage.
Kwik-Sil™ Applications
Kwik-Sil™ silicones are based on technology with vinyl terminated siloxane and platinum complex catalysts. In order to cure at room temperature, special cross linkers and high catalyst concentration are used. Although the high concentration of catalysts makes these products more costly than traditional RTV silicones and less attractive for general usage, they provide an excellent value in applications for the biological research field.

Benefits
Applications
|
  |
Kwik-Sil and Kwik-Cast silicone adhesives have very low toxicity before, during and after curing. The by-product of curing is a small amount of hydrogen gas, which is much less toxic to cells than acetic acid or alcohol from traditional RTV silicone systems.
Kwik-Sil and Kwik-Cast curing speed is hundreds of times faster than traditional RTV silicones. A curing time of a few minutes at room temperature is especially useful for encapsulation of live tissue or implanting into a live animal. Unlike many vinyl-based silicones in which the platinum complex catalysts are easily poisoned by contamination from amines and animal tissue, Kwik-Sil and Kwik-Cast surgical adhesives are not sensitive to contamination from animal tissue.
Kwik-Sil™ is a translucent, medium-viscosity silicone adhesive, developed for chronic peripheral nerve studies such as anterograde tracing with fluorescent indicators or electrode recording. Good adhesion and mechanical properties (tear strength and elongation) allow days of study without breaking of the bonding. Curing speed is very reproducible.
Kwik-Cast™ is a very low viscosity silicone sealant developed to embed peripheral nerves with electrodes for acute multi-fiber recordings. It flows easily, filling the small spaces around the nerve and leaving no channels through which peritoneal fluid can travel and thus short the nerve/electrode contact. Equally important is the ability of the material to flow into itself and create one continuous mass from underneath the nerve all the way to the top of the nerve/electrode contact to ensure long-term recording stability. Kwik-Cast is color-coded to make the mixing foolproof. The catalyst is yellow and the base is blue. When uniformly mixed, it is green. Kwik-Cast can be applied and cured underneath mineral oil. After recording, electrodes are easily recovered due to the low tear strength. The slight longer curing time (approx. 3 minutes) makes it more suitable for stationary preparations and in vitro tissue studies.
KWIK-SIL Videos
Kwik-Sil™ vs Kwik-Cast™
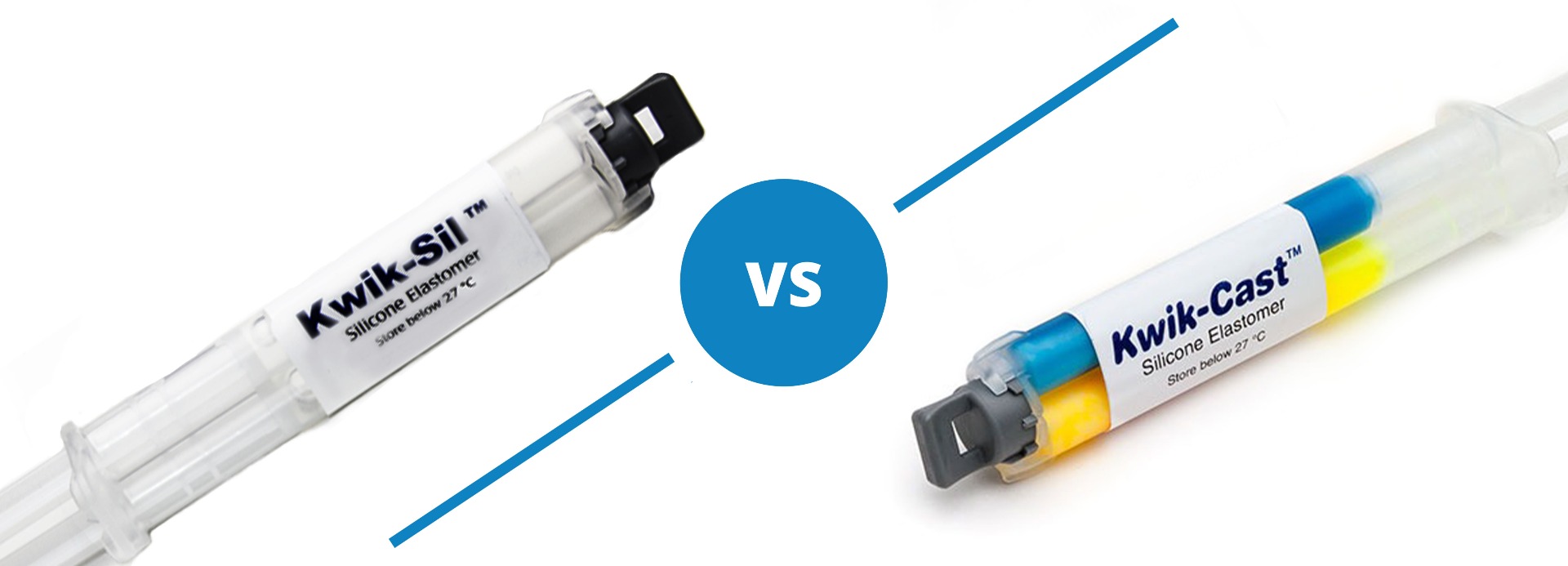
Both Kwik-Sil and Kwik-Cast are silicone adhesives, but they are a little different.
Kwik-Sil™ is a translucent, medium-viscosity silicone adhesive, developed for chronic peripheral nerve studies such as anterograde tracing with fluorescent indicators or electrode recording. Good adhesion and mechanical properties (tear strength and elongation) allow days of study without breaking of the bonding. Curing speed is very reproducible.
Kwik-Cast™ is a very low viscosity silicone sealant developed to embed peripheral nerves with electrodes for acute multi-fiber recordings. It flows easily, filling the small spaces around the nerve and leaving no channels through which peritoneal fluid can travel and thus short the nerve/electrode contact. Equally important is the ability of the material to flow into itself and create one continuous mass from underneath the nerve all the way to the top of the nerve/electrode contact to ensure long-term recording stability. Kwik-Cast is color-coded to make the mixing foolproof. The catalyst is yellow and the base is blue. When uniformly mixed, it is green. Kwik-Cast can be applied and cured underneath mineral oil. After recording, electrodes are easily recovered due to the low tear strength. The slight longer curing time (approx. 3 minutes) makes it more suitable for stationary preparations and in vitro tissue studies.
Specifications
KWIK-SIL Silicone Adhesive Compound (two 5-mL syringes)
| Kwik-Sil | |
| MIX RATIO | 1 to 1 |
| WORKING TIME | < 5 minutes* |
| SETTING TIME (ROOM TEMP., 1:1 RATIO) | 5–10 minutes** |
| CURE TIME | ~15 minutes |
| VISCOSITY, CPS | 15,000 |
| GUARANTEED SHELF LIFE AT 23 °C | 6 months |
| VOLUME | 5 mL |
| NUMBER OF MIXING TIP | 10 |
| DEAD VOLUME OF THE MIXING TIP | <0.12 mL |
| AFTER CURING 24 HOURS: | |
| TEAR STRENGTH, PPI | 90 |
| ELONGATION % | 650 |
| DUROMETER (SHORE A-2) | 30 |
| COLOR | translucent |
| VOLUME RESISTIVITY, W/CM | 1x1015 |
* 3 minutes average with about 90 seconds of liquidity
** no longer mixable at this point
Accessories
600022
Replacement Mixing Tips for KWIK-SIL and KWIK-CAST. Package of 10. For use with the ...
Citations
Pisano, Thomas & Hoag, Austin & Dhanerawala, Zahra & Guariglia, Sara & Jung, Caroline & Boele, Henk-Jan & Seagraves, Kelly & Verpeut, Jessica & Wang, Samuel. (2022). Automated high-throughput mouse transsynaptic viral tracing using iDISCO+ tissue clearing, light-sheet microscopy, and BrainPipe. STAR Protocols. 3. 101289. 10.1016/j.xpro.2022.101289.
Ng, D., Pinharanda, A., Vogt, M. C., Litwin-Kumar, A., Stearns, K., Thopte, U., Cannavo, E., Enikolopov, A., Fiederling, F., Kosmidis, S., Noro, B., Rodrigues-Vaz, I., Shayya, H., Andolfatto, P., Peterka, D. S., Tabachnik, T., D’Armiento, J., Goldklang, M., & Bendesky, A. (2021). WHotLAMP: A simple, inexpensive, and sensitive molecular test for the detection of SARS-CoV-2 in saliva. PLoS ONE, 16(9). https://doi.org/10.1371/JOURNAL.PONE.0257464
McKillop, L. E., Fisher, S. P., Milinski, L., Krone, L. B., & Vyazovskiy, V. v. (2021). Diazepam effects on local cortical neural activity during sleep in mice. Biochemical Pharmacology, 191. https://doi.org/10.1016/J.BCP.2021.114515
Fiederling, F., Hammond, L. A., Ng, D., Mason, C., & Dodd, J. (2021). Tools for efficient analysis of neurons in a 3D reference atlas of whole mouse spinal cord. Cell Reports Methods, 1(5). https://doi.org/10.1016/J.CRMETH.2021.100074
Ausra, J., Wu, M., Zhang, X., Vázquez-Guardado, A., Skelton, P., Peralta, R., Avila, R., Murickan, T., Haney, C. R., Huang, Y., Rogers, J. A., Kozorovitskiy, Y., & Gutruf, P. (2021). Wireless, battery-free, subdermally implantable platforms for transcranial and long-range optogenetics in freely moving animals. Proceedings of the National Academy of Sciences of the United States of America, 118(30). https://doi.org/10.1073/PNAS.2025775118/SUPPL_FILE/PNAS.2025775118.SM05.MOV
Khan, I., Prabhakar, A., Delepine, C., Tsang, H., Pham, V., & Sur, M. (2021). A low-cost 3D printed microfluidic bioreactor and imaging chamber for live-organoid imaging. Biomicrofluidics, 15(2), 024105. https://doi.org/10.1063/5.0041027
Skyberg, R., Sun, C., & Hill, D. L. (2020). Selective Removal of Sodium Salt Taste Disrupts the Maintenance of Dendritic Architecture of Gustatory Relay Neurons in the Mouse Nucleus of the Solitary Tract. ENeuro, 7(5). https://doi.org/10.1523/ENEURO.0140-20.2020
Grover, D., Katsuki, T., Li, J., Dawkins, T. J., & Greenspan, R. J. (2020). Imaging brain activity during complex social behaviors in Drosophila with Flyception2. Nature Communications, 11(1). https://doi.org/10.1038/s41467-020-14487-7
Benson, C. A., Fenrich, K. K., Olson, K. L., Patwa, S., Bangalore, L., Waxman, S. G., & Tan, A. M. (2020). Dendritic spine dynamics after peripheral nerve injury: An intravital structural study. Journal of Neuroscience, 40(22), 4297. https://doi.org/10.1523/JNEUROSCI.2858-19.2020
He, F., He, F., Sullender, C. T., Zhu, H., Williamson, M. R., Li, X., … Luan, L. (2020). Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes. Science Advances, 6(21), 1933. https://doi.org/10.1126/sciadv.aba1933
Solarana, K., Ye, M., Gao, Y.-R., Rafi, H., & Hammer, D. X. (2020). Longitudinal multimodal assessment of neurodegeneration and vascular remodeling correlated with signal degradation in chronic cortical silicon microelectrodes. Neurophotonics, 7(01), 1. https://doi.org/10.1117/1.nph.7.1.015004
Gill, J. P., & Chiel, H. J. (2020). Rapid adaptation to changing mechanical load by ordered recruitment of identified motor neurons. ENeuro, 7(3). https://doi.org/10.1523/ENEURO.0016-20.2020
McPheeters, M. T., Blackburn, B. J., Dupps, W. J., Rollins, A. M., & Jenkins, M. W. (2020). Genetically encoded calcium indicators for in situ functional studies of corneal nerves. Investigative Ophthalmology and Visual Science, 61(13). https://doi.org/10.1167/IOVS.61.13.10
Pernici, C. D., Rowe, R. K., Doughty, P. T., Madadi, M., Lifshitz, J., & Murray, T. A. (2020). Longitudinal optical imaging technique to visualize progressive axonal damage after brain injury in mice reveals responses to different minocycline treatments. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-64783-x
Koch, P. F., Cottone, C., Adam, C. D., Ulyanova, A. V., Russo, R. J., Weber, M. T., … Wolf, J. A. (2020). Traumatic Brain Injury Preserves Firing Rates But Disrupts Laminar Oscillatory Coupling and Neuronal Entrainment in Hippocampal CA1. ENeuro, 7(5). https://doi.org/10.1523/ENEURO.0495-19.2020
Shuman, T., Aharoni, D., Cai, D. J., Lee, C. R., Chavlis, S., Page-Harley, L., … Golshani, P. (2020). Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nature Neuroscience, 23(2), 229–238. https://doi.org/10.1038/s41593-019-0559-0
Fan Y, Jiang E, Hahka T, Chen QH, Yan J, Shan Z. Orexin A increases sympathetic nerve activity through promoting expression of proinflammatory cytokines in Sprague Dawley rats. Acta Physiol (Oxf). 2017 Sep 5. doi: 10.1111/apha.12963. https://www.ncbi.nlm.nih.gov/pubmed/28872777 .
Yang, Z., Xie, W., Ju, F., khan, A., & Zhang, S. (2017). In vivo two-photon imaging reveals a role of progesterone in reducing axonal dieback after spinal cord injury in mice. Neuropharmacology, 116, 30–37. http://doi.org/10.1016/j.neuropharm.2016.12.007
Paoli, M., Andrione, M., & Haase, A. (2017). Imaging Techniques in Insects (pp. 471–519). http://doi.org/10.1007/978-1-4939-6725-4_15
Madden, C. J., Santos da Conceicao, E. P., & Morrison, S. F. (2017). Vagal afferent activation decreases brown adipose tissue (BAT) sympathetic nerve activity and BAT thermogenesis. Temperature, 4(1), 89–96. http://doi.org/10.1080/23328940.2016.1257407
Laszt?czi, B., & Klausberger, T. (2017). Distinct gamma oscillations in the distal dendritic fields of the dentate gyrus and the CA1 area of mouse hippocampus. Brain Structure and Function, 1–11. http://doi.org/10.1007/s00429-017-1421-3
Kawada, T., Turner, M. J., Shimizu, S., Fukumitsu, M., Kamiya, A., & Sugimachi, M. (2017). Aortic depressor nerve stimulation does not impede the dynamic characteristics of the carotid sinus baroreflex in normotensive or spontaneously hypertensive rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 312(5). Retrieved from http://ajpregu.physiology.org/content/312/5/R787
Bassi, G. S., Dias, D. P. M., Franchin, M., Talbot, J., Reis, D. G., Menezes, G. B., … Kanashiro, A. (2017). Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain, Behavior, and Immunity, 64, 330–343. http://doi.org/10.1016/j.bbi.2017.04.003
Dai, X., Zhou, W., Gao, T., Liu, J., & Lieber, C. M. (2016). Supplementary Information for: Three-dimensional mapping and regulation of action potential propagation in nanoelectronics innervated tissues Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. https://doi.org/10.1038/NNANO.2016.96
Balkowiec, A., Kunze, D. L., & Katz, D. M. (n.d.). Brain-Derived Neurotrophic Factor Acutely Inhibits AMPA-Mediated Currents in Developing Sensory Relay Neurons.
Bechtold, A. G., & Scheuer, D. A. (n.d.). Glucocorticoids act in the dorsal hindbrain to modulate baroreflex control of heart rate.
Gao, L., Zhu, Z., Zucker, I. H., & Wang, W. (n.d.). Cardiac sympathetic afferent stimulation impairs baroreflex control of renal sympathetic nerve activity in rats.
Kakegawa, W., Miyazaki, T., Kohda, K., Matsuda, K., Emi, K., Motohashi, J., … Yuzaki, M. (n.d.). The N-Terminal Domain of GluD2 (GluR?2) Recruits Presynaptic Terminals and Regulates Synaptogenesis in the Cerebellum In Vivo. http://doi.org/10.1523/JNEUROSCI.6013-08.2009
Luckett, B. S., Frielle, J. L., Wolfgang, L., & Stocker, S. D. (n.d.). Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin.
Mizuno, M., Murphy, M. N., Mitchell, J. H., & Smith, S. A. (n.d.). Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats.
Salgado-Commissariat, D., & Alkadhi, K. A. Serotonin inhibits epileptiform discharge by activation of 5-HT1A receptors in CA1 pyramidal neurons. Neuropharmacology, 36(11–12), 1705–12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9517442
Wang, W.-Z., Gao, L., Wang, H.-J., Zucker, I. H., & Wang, W. (n.d.). Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT 1 receptors in heart failure.
Xie, C., Sachs, J. R., & Wang, D. H. (n.d.). Interdependent Regulation of Afferent Renal Nerve Activity and Renal Function: Role of Transient Receptor Potential Vanilloid Type 1, Neurokinin 1, and Calcitonin Gene-Related Peptide Receptors. http://doi.org/10.1124/jpet.108.136374
Yamamoto, K., Kawada, T., Kamiya, A., Takaki, H., Miyamoto, T., Sugimachi, M., & Sunagawa, K. (n.d.). Muscle mechanoreflex induces the pressor response by resetting the arterial baroreflex neural arc.
Thompson, Z. S., Rijal, N. P., Jarvis, D., Edwards, A., & Bhattarai, N. (2016). Synthesis of Keratin-based Nanofiber for Biomedical Engineering. Journal of Visualized Experiments, (108), e53381–e53381. http://doi.org/10.3791/53381
Schwarz, M. K., Scherbarth, A., Sprengel, R., Engelhardt, J., Theer, P., Giese, G., … Tomancak, P. (2015). Fluorescent-Protein Stabilization and High-Resolution Imaging of Cleared, Intact Mouse Brains. PLOS ONE, 10(5), e0124650. http://doi.org/10.1371/journal.pone.0124650
Farrar, M. J., & Schaffer, C. B. (2014). A Procedure for Implanting a Spinal Chamber for Longitudinal In Vivo Imaging of the Mouse Spinal Cord. Journal of Visualized Experiments, (94), e52196–e52196. http://doi.org/10.3791/52196
Kawada, T., Li, M., Zheng, C., Shimizu, S., Uemura, K., Turner, M. J., … Sugimachi, M. (2014). Chronic vagal nerve stimulation improves baroreflex neural arc function in heart failure rats Chronic vagal nerve stimulation im- proves baroreflex neural arc function in heart failure rats. J Appl Physiol, 116, 1308–1314. http://doi.org/10.1152/japplphysiol.00140.2014
Kawada, T., Li, M., Zheng, C., Shimizu, S., Uemura, K., Turner, M. J., … Sugimachi, M. (2014). Chronic vagal nerve stimulation improves baroreflex neural arc function in heart failure rats. Journal of Applied Physiology (Bethesda, Md.?: 1985), 116(10), 1308–14. http://doi.org/10.1152/japplphysiol.00140.2014
Kislin, M., Mugantseva, E., Molotkov, D., Kulesskaya, N., Khirug, S., Kirilkin, I., … Khiroug, L. (2014). Flat-floored Air-lifted Platform: A New Method for Combining Behavior with Microscopy or Electrophysiology on Awake Freely Moving Rodents. Journal of Visualized Experiments, (88), e51869–e51869. http://doi.org/10.3791/51869
Rodríguez-Contreras, A., Shi, L., & Fu, B. M. (2014). A Method to Make a Craniotomy on the Ventral Skull of Neonate Rodents. Journal of Visualized Experiments, (87), e51350–e51350. http://doi.org/10.3791/51350
Roome, C. J., & Kuhn, B. (2014). Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology. Frontiers in Cellular Neuroscience, 8, 379. http://doi.org/10.3389/fncel.2014.00379
Zimmermann, J. B., & Jackson, A. (2014). Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Frontiers in Neuroscience, 8, 87. http://doi.org/10.3389/fnins.2014.00087
Fenrich, K. K., Weber, P., Rougon, G., & Debarbieux, F. (2013). Implanting Glass Spinal Cord Windows in Adult Mice with Experimental Autoimmune Encephalomyelitis. Journal of Visualized Experiments, (82), e50826–e50826. http://doi.org/10.3791/50826
Woolley, A. J., Desai, H. A., Gaire, J., Ready, A. L., & Otto, K. J. (2013). Intact Histological Characterization of Brain-implanted Microdevices and Surrounding Tissue. Journal of Visualized Experiments, (72), e50126–e50126. http://doi.org/10.3791/50126
Fenrich, K. K., Weber, P., Hocine, M., Zalc, M., Rougon, G., & Debarbieux, F. (2012). Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. The Journal of Physiology, 590(Pt 16), 3665–75. http://doi.org/10.1113/jphysiol.2012.230532
Gage, G. J., Stoetzner, C. R., Richner, T., Brodnick, S. K., Williams, J. C., & Kipke, D. R. (2012). Surgical implantation of chronic neural electrodes for recording single unit activity and electrocorticographic signals. Journal of Visualized Experiments?: JoVE, (60), e3565. http://doi.org/10.3791/3565
Morgan, S. J., & Paolini, A. G. (2012). Behavioral Determination of Stimulus Pair Discrimination of Auditory Acoustic and Electrical Stimuli Using a Classical Conditioning and Heart-rate Approach. Journal of Visualized Experiments, (64), e3598–e3598. http://doi.org/10.3791/3598
Shi, Z., Chen, W.-W., Xiong, X.-Q., Han, Y., Zhou, Y.-B., Zhang, F., … Zhu, G.-Q. (2012). Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol, 112, 1008–1014. http://doi.org/10.1152/japplphysiol.01164.2011
Silbering, A. F., Bell, R., Galizia, C. G., & Benton, R. (2012). Calcium Imaging of Odor-evoked Responses in the Drosophila Antennal Lobe. Journal of Visualized Experiments, (60), e2976–e2976. http://doi.org/10.3791/2976
Harris, J. P., Capadona, J. R., Miller, R. H., Healy, B. C., Shanmuganathan, K., Rowan, S. J., … Tyler, D. J. (2011). Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies. Journal of Neural Engineering, 8(6), 66011. http://doi.org/10.1088/1741-2560/8/6/066011
Cullins, M. J., & Chiel, H. J. (2010). Electrode Fabrication and Implantation in Aplysia californica for Multi-channel Neural and Muscular Recordings in Intact, Freely Behaving Animals. Journal of Visualized Experiments, (40), e1791–e1791. http://doi.org/10.3791/1791
Huber, D. A., & Schreihofer, A. M. (2010). Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. The Journal of Physiology, 588(9), 1515–1525. http://doi.org/10.1113/jphysiol.2009.186387
Kamiya, A., Kawada, T., Mizuno, M., Shimizu, S., & Sugimachi, M. (2010). Parallel resetting of arterial baroreflex control of renal and cardiac sympathetic nerve activities during upright tilt in rabbits. Am J Physiol Heart Circ Physiol, 298, 1966–1975. http://doi.org/10.1152/ajpheart.00340.2009
Morgan, D. A., & Rahmouni, K. (2010). Differential effects of insulin on sympathetic nerve activity in agouti obese mice. Journal of Hypertension, 28(9), 1913–9. http://doi.org/10.1097/HJH.0b013e32833c2289
Battaglia, F. P., Kalenscher, T., Cabral, H., Winkel, J., Bos, J., Manuputy, R., … Pennartz, C. (2009). The Lantern: an ultra-light micro-drive for multi-tetrode recordings in mice and other small animals. Journal of Neuroscience Methods, 178(2), 291–300. http://doi.org/10.1016/j.jneumeth.2008.12.024
Bechtold, A. G., Patel, G., Hochhaus, G., & Scheuer, D. A. (2009). Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am J Physiol Regul Integr Comp Physiol, 296, 1445–1454. http://doi.org/10.1152/ajpregu.00095.2008
Dombeck, D. A., Graziano, M. S., & Tank, D. W. (2009). Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. The Journal of Neuroscience?: The Official Journal of the Society for Neuroscience, 29(44), 13751–60. http://doi.org/10.1523/JNEUROSCI.2985-09.2009
Haehnel, M., Froese, A., & Menzel, R. (2009). In vivo Ca2+- Imaging of Mushroom Body Neurons During Olfactory Learning in the Honey Bee. Journal of Visualized Experiments, (30), e1353–e1353. http://doi.org/10.3791/1353
Jackson, N., & Muthuswamy, J. (2008). Artificial dural sealant that allows multiple penetrations of implantable brain probes. Journal of Neuroscience Methods, 171(1), 147–52. http://doi.org/10.1016/j.jneumeth.2008.02.018
Spitler, K. M., & Gothard, K. M. (2008). A removable silicone elastomer seal reduces granulation tissue growth and maintains the sterility of recording chambers for primate neurophysiology. Journal of Neuroscience Methods, 169(1), 23–6. http://doi.org/10.1016/j.jneumeth.2007.11.026
Pan, Y.-X., Gao, L., Wang, W.-Z., Zheng, H., Liu, D., Patel, K. P., … Wang, W. (2007). Exercise Training Prevents Arterial Baroreflex Dysfunction in Rats Treated With Central Angiotensin II. Hypertension, 49(3).
Kurata, K., Heino, T. J., Higaki, H., & Väänänen, H. K. (2006). Bone Marrow Cell Differentiation Induced by Mechanically Damaged Osteocytes in 3D Gel-Embedded Culture. Journal of Bone and Mineral Research, 21(4), 616–625. http://doi.org/10.1359/jbmr.060106
Sachse, S., Peele, P., Silbering, A. F., Gühmann, M., & Galizia, C. G. (2006). Role of histamine as a putative inhibitory transmitter in the honeybee antennal lobe. Frontiers in Zoology, 3(1), 22. http://doi.org/10.1186/1742-9994-3-22
Barrett, C. J., Guild, S.-J., Ramchandra, R., & Malpas, S. C. (2005). Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension, 46(1), 168–72. http://doi.org/10.1161/01.HYP.0000168047.09637.d4
Gao, L., Wang, W., Li, Y.-L., Schultz, H. D., Liu, D., Cornish, K. G., & Zucker, I. H. (2005). Sympathoexcitation by central ANG II: Roles for AT 1 receptor upregulation and NAD(P)H oxidase in RVLM Sympatho- excitation by central ANG II: Roles for AT 1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol, 288, 2271–2279. http://doi.org/10.1152/ajpheart.00949.2004
Barann, M., Dilger, J. P., Bönisch, H., Göthert, M., Dybek, A., & Urban, B. W. (2000). Inhibition of 5-HT3 receptors by propofol: equilibrium and kinetic measurements. Neuropharmacology, 39(6), 1064–74. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10727717
Mire, P., Nasse, J., & Venable-Thibodeaux, S. (2000). Gap junctional communication in the vibration-sensitive response of sea anemones. Hearing Research, 144(1–2), 109–23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10831870
Cannon, S. C., & Corey, D. P. (1993). Loss of Na+ channel inactivation by anemone toxin (ATX II) mimics the myotonic state in hyperkalaemic periodic paralysis. The Journal of Physiology, 466, 501–20. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1175489&tool=pmcentrez&rendertype=abstract
RelatedItems
KWIK-CAST-S
Kwik-Cast™ Sterile Packed low toxicity adhesives for live tissues
Includ...






Request
Catalogue
Chat
Print