

FD35-100
FluoroDish, Glass Bottom, Clear Wall, 35 mm, 23 mm Well, Individually Packed, Sterile, 100/bx
- Overview
- Specifications
- Accessories
- Citations
- Related Products
Overview

There are 1 images available to view - click to enlarge and scroll through the product gallery.
FluoroDish Datasheet
/ Download as PDF
- Optical quality glass bottom for better imaging quality
- Low sample volume for expensive chemicals
- Lowest access angle for micropipette
- Low toxicity adhesive for embryo research
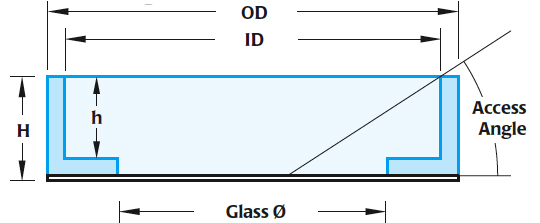
Measurements
- ID: 33mm
- OD: 35.5mm
- Glass Diameter Φ: 23.5mm
- height (inside): 7.8mm
- Height (outside): 9mm
- Access angle: 29 degrees
Exceptional Imaging Quality
WPI’s FluoroDish™ tissue culture dishes are now available in a larger range of sizes and coatings. These polycarbonate dishes provide exceptional imaging quality for many applications requiring the use of inverted microscopes such as high resolution image analysis, microinjection and electrophysical recording of fluorescent-tagged cells. Taking advantage of WPI’s extensive experience with low toxicity adhesives, FluoroDish uses a specially formulated adhesive that is optically clear, durable and with extremely low toxicity. Tests by an independent laboratory have shown that the 96-hour surviving rate of embryos is 100% when kept in FluoroDish, substantially better than some other brands. The bottom glass has superior UV transmission (30% transmission at 300 nm, compared to less than 7% for the most popular German glass). Stringent quality control ensures that glass thickness stays within the 0.17 ±0.01 mm range.
Excellent for both classical and fluorescence microscopy
Each WPI dish has a flat (0.17 mm thick) optical quality glass bottom, allowing the use of a short objective working distance, large numerical aperture (NA), and a high magnification (up to 100x). The larger NA and higher magnification provide superior quality imaging for both classical and fluorescence microscopy. Higher effective NA yields brighter images for fluorescence and higher resolution in Image Analysis. The glass bottom permits the use of immersion objectives with medium such as water, glycerin or oil for the highest magnification possible. To optimize heat-exchange, WPI’s glass-bottom dish is designed to be flush (flat) with the microscope stage or heating unit, therefore eliminating the air gap that exists with modified plastic dishes in which a glass cover slip has been inserted.
Materials
An inner well is created within the dish by the glass bottom and the tissue culture grade polystyrene which forms the sides of the dish. All WPI dishes have the advantages of low toxicity and good UV transmission bottom glass. They are individually packed and gamma sterilized.
Features
The 35 mm dish has outside dimensions similar to that of a Corning 35 mm dish and has ø23.5 mm glass window. Most heaters and perfusion adapters designed for the Corning 35 mm dish will also fit this dish. Certain types of cell lines (e.g., PC3 and HEK) adhere well to the uncoated glass bottom dish. The users can also apply to the uncoated dish any special coating that is best for their cell line.
Human neural stem cells freshly dispersed by Accutase seeded on the glass coverslip bottom of a WPI Fluorodish, timelapse-imaged over a period of 6 days.
Specifications
Accessories
Citations
Roscioli, E., Germanova, T. E., Smith, C. A., Embacher, P. A., Erent, M., Thompson, A. I., … McAinsh, A. D. (2020). Ensemble-Level Organization of Human Kinetochores and Evidence for Distinct Tension and Attachment Sensors. Cell Reports, 31(4). https://doi.org/10.1016/j.celrep.2020.107535
Forrester, A., Rathjen, S. J., Daniela Garcia-Castillo, M., Bachert, C., Couhert, A., Tepshi, L., … Johannes, L. (2020). Functional dissection of the retrograde Shiga toxin trafficking inhibitor Retro-2. Nature Chemical Biology, 16(3), 327–336. https://doi.org/10.1038/s41589-020-0474-4
Shah, A., Plaza-Sirvent, C., Weinert, S., Buchbinder, J. H., Lavrik, I. N., Mertens, P. R., … Lindquist, J. A. (2020). Yb-1 mediates tnf-induced pro-survival signaling by regulating nf-κb activation. Cancers, 12(8), 1–12. https://doi.org/10.3390/cancers12082188
Samassa, F., Ferrari, M. L., Husson, J., Mikhailova, A., Porat, Z., Sidaner, F., … Phalipon, A. (2020). Shigella impairs human T lymphocyte responsiveness by hijacking actin cytoskeleton dynamics and T cell receptor vesicular trafficking. Cellular Microbiology, 22(5). https://doi.org/10.1111/cmi.13166
Andersen, J. P., Zhang, J., Sun, H., Liu, X., Liu, J., Nie, J., & Shi, Y. (2020). Aster-B coordinates with Arf1 to regulate mitochondrial cholesterol transport. Molecular Metabolism, 42, 101055. https://doi.org/10.1016/j.molmet.2020.101055
Mateus, R., Holtzer, L., Seum, C., Hadjivasiliou, Z., Dubois, M., Jülicher, F., & Gonzalez-Gaitan, M. (2020). BMP Signaling Gradient Scaling in the Zebrafish Pectoral Fin. Cell Reports, 30(12), 4292-4302.e7. https://doi.org/10.1016/j.celrep.2020.03.024
Ibrahim, A. F. M., Shen, L., Tatham, M. H., Dickerson, D., Prescott, A. R., Abidi, N., … Hay, R. T. (2020). Antibody RING-Mediated Destruction of Endogenous Proteins. Molecular Cell, 79(1), 155-166.e9. https://doi.org/10.1016/j.molcel.2020.04.032
Fore, S., Acuña-Hinrichsen, F., Mutlu, K. A., Bartoszek, E. M., Serneels, B., Faturos, N. G., … Yaksi, E. (2020). Functional properties of habenular neurons are determined by developmental stage and sequential neurogenesis. Science Advances, 6(36). https://doi.org/10.1126/sciadv.aaz3173
Alijevic, O., Bignucolo, O., Hichri, E., Peng, Z., Kucera, J. P., & Kellenberger, S. (2020). Slowing of the Time Course of Acidification Decreases the Acid-Sensing Ion Channel 1a Current Amplitude and Modulates Action Potential Firing in Neurons. Frontiers in Cellular Neuroscience, 14, 41. https://doi.org/10.3389/fncel.2020.00041
Van Der Meulen, K. L., Vöcking, O., Weaver, M. L., Meshram, N. N., & Famulski, J. K. (2020). Spatiotemporal Characterization of Anterior Segment Mesenchyme Heterogeneity During Zebrafish Ocular Anterior Segment Development. Frontiers in Cell and Developmental Biology, 8. https://doi.org/10.3389/fcell.2020.00379
Palumbo, F., Serneels, B., Pelgrims, R., & Yaksi, E. (2020). The Zebrafish Dorsolateral Habenula Is Required for Updating Learned Behaviors. Cell Reports, 32(8). https://doi.org/10.1016/j.celrep.2020.108054
Bolado-Carrancio, A., Rukhlenko, O. S., Nikonova, E., Tsyganov, M. A., Wheeler, A., Garcia-Munoz, A., … Kholodenko, B. N. (2020). Periodic propagating waves coordinate rhogtpase network dynamics at the leading and trailing edges during cell migration. ELife, 9, 1–34. https://doi.org/10.7554/eLife.58165
Ecke, M., Prassler, J., Tanribil, P., Müller-Taubenberger, A., Körber, S., Faix, J., & Gerisch, G. (2020). Formins specify membrane patterns generated by propagating actin waves. Molecular Biology of the Cell, 31(5), 373–385. https://doi.org/10.1091/mbc.E19-08-0460
Mulier, M., Van Ranst, N., Corthout, N., Munck, S., Vanden Berghe, P., Vriens, J., … Moilanen, L. (2020). Upregulation of TRPM3 in nociceptors innervating inflamed tissue. ELife, 9. https://doi.org/10.7554/eLife.61103
Rohani, L., Borys, B. S., Razian, G., Naghsh, P., Liu, S., Johnson, A. A., … Rancourt, D. E. (2020). Stirred suspension bioreactors maintain naïve pluripotency of human pluripotent stem cells. Communications Biology, 3(1). https://doi.org/10.1038/s42003-020-01218-3
Surewicz, W., & Babinchak, W. (2020). Studying Protein Aggregation in the Context of Liquid-liquid Phase Separation Using Fluorescence and Atomic Force Microscopy, Fluorescence and Turbidity Assays, and FRAP. BIO-PROTOCOL, 10(2). https://doi.org/10.21769/bioprotoc.3489
Gao, X., Jiang, Y., Lin, Y., Kim, K. H., Fang, Y., Yi, J., … Tian, B. (2020). Structured silicon for revealing transient and integrated signal transductions in microbial systems. Science Advances, 6(7), 2760. https://doi.org/10.1126/sciadv.aay2760
Shao, W., Yang, J., He, M., Yu, X. Y., Lee, C. H., Yang, Z., … Shi, S. H. (2020). Centrosome anchoring regulates progenitor properties and cortical formation. Nature, 580(7801), 106–112. https://doi.org/10.1038/s41586-020-2139-6
Chronopoulos, A., Thorpe, S. D., Cortes, E., Lachowski, D., Rice, A. J., Mykuliak, V. V., … del Río Hernández, A. E. (2020). Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nature Materials, 19(6), 669–678. https://doi.org/10.1038/s41563-019-0567-1
Beletkaia, E., Dashtbozorg, B., Jansen, R. G., Ruers, T. J. M., & Offerhaus, H. L. (2020). Nonlinear multispectral imaging for tumor delineation. Journal of Biomedical Optics, 25(09). https://doi.org/10.1117/1.jbo.25.9.096001
Ndao, O., Puech, P. H., Bérard, C., Limozin, L., Rabhi, S., Azas, N., … Dumètre, A. (2020). Dynamics of Toxoplasma gondii Oocyst Phagocytosis by Macrophages. Frontiers in Cellular and Infection Microbiology, 10. https://doi.org/10.3389/fcimb.2020.00207
Otis, J. P., & Farber, S. A. (2016). High-fat Feeding Paradigm for Larval Zebrafish: Feeding, Live Imaging, and Quantification of Food Intake. Journal of Visualized Experiments, (116), e54735–e54735. http://doi.org/10.3791/54735
Arnold, W. D., Sheth, K. A., Wier, C. G., Kissel, J. T., Burghes, A. H., & Kolb, S. J. (2015). Electrophysiological Motor Unit Number Estimation (MUNE) Measuring Compound Muscle Action Potential (CMAP) in Mouse Hindlimb Muscles. Journal of Visualized Experiments, (103), e52899–e52899. http://doi.org/10.3791/52899
Gindrat, A.-D., Quairiaux, C., Britz, J., Brunet, D., Lanz, F., Michel, C. M., & Rouiller, E. M. (2015). Whole-scalp EEG mapping of somatosensory evoked potentials in macaque monkeys. Brain Structure & Function, 220(4), 2121–42. http://doi.org/10.1007/s00429-014-0776-y
Lee, E., Hong, J., Park, Y.-G., Chae, S., Kim, Y., Kim, D., … Sirota, A. (2015). Left brain cortical activity modulates stress effects on social behavior. Scientific Reports, 5, 13342. http://doi.org/10.1038/srep13342
Nunes, P., Guido, D., & Demaurex, N. (2015). Measuring Phagosome pH by Ratiometric Fluorescence Microscopy. Journal of Visualized Experiments, (106), e53402–e53402. http://doi.org/10.3791/53402
Pothoven, K. L., Norton, J. E., Hulse, K. E., Suh, L. A., Carter, R. G., Rocci, E., … Schleimer, R. P. (2015). Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. Journal of Allergy and Clinical Immunology, 136(3), 737–746.e4. http://doi.org/10.1016/j.jaci.2015.01.043
Rees, M. D., & Thomas, S. R. (2015). Using Cell-substrate Impedance and Live Cell Imaging to Measure Real-time Changes in Cellular Adhesion and De-adhesion Induced by Matrix Modification. Journal of Visualized Experiments, (96), e52423–e52423. http://doi.org/10.3791/52423
Srinivasan, B., Kolli, A. R., Esch, M. B., Abaci, H. E., Shuler, M. L., & Hickman, J. J. (2015). TEER measurement techniques for in vitro barrier model systems. Journal of Laboratory Automation, 20(2), 107–26. http://doi.org/10.1177/2211068214561025
Steinritz, D., Schmidt, A., Balszuweit, F., Thiermann, H., Ibrahim, M., Bölck, B., & Bloch, W. (2015). Assessment of Endothelial Cell Migration After Exposure to Toxic Chemicals. Journal of Visualized Experiments, (101), e52768–e52768. http://doi.org/10.3791/52768
Al-Sadi, R., Ye, D., Boivin, M., Guo, S., Hashimi, M., Ereifej, L., … Yaguchi, A. (2014). Interleukin-6 Modulation of Intestinal Epithelial Tight Junction Permeability Is Mediated by JNK Pathway Activation of Claudin-2 Gene. PLoS ONE, 9(3), e85345. http://doi.org/10.1371/journal.pone.0085345
Alcolado, N. G., Conrad, D. J., Poroca, D., Li, M., Alshafie, W., Chappe, F. G., … Chappe, V. M. (2014). Cystic fibrosis transmembrane conductance regulator dysfunction in VIP knockout mice. American Journal of Physiology. Cell Physiology, 307(2), C195-207. http://doi.org/10.1152/ajpcell.00293.2013
Avila, I., & Lin, S.-C. (2014). Motivational salience signal in the basal forebrain is coupled with faster and more precise decision speed. PLoS Biology, 12(3), e1001811. http://doi.org/10.1371/journal.pbio.1001811
Blanchard, E., Zlock, L., Lao, A., Mika, D., Namkung, W., Xie, M., … Richter, W. (2014). Anchored PDE4 regulates chloride conductance in wild-type and ΔF508-CFTR human airway epithelia. FASEB Journal?: Official Publication of the Federation of American Societies for Experimental Biology, 28(2), 791–801. http://doi.org/10.1096/fj.13-240861
Brayden, D. J., & Walsh, E. (2014). Efficacious intestinal permeation enhancement induced by the sodium salt of 10-undecylenic acid, a medium chain fatty acid derivative. The AAPS Journal, 16(5), 1064–76. http://doi.org/10.1208/s12248-014-9634-3
Henson, H. E., Parupalli, C., Ju, B., & Taylor, M. R. (2014). Functional and genetic analysis of choroid plexus development in zebrafish. Frontiers in Neuroscience, 8, 364. http://doi.org/10.3389/fnins.2014.00364
Horst, M., Milleret, V., Nötzli, S., Madduri, S., Sulser, T., Gobet, R., & Eberli, D. (2014). Increased porosity of electrospun hybrid scaffolds improved bladder tissue regeneration. Journal of Biomedical Materials Research Part A, 102(7), 2116–2124. http://doi.org/10.1002/jbm.a.34889
Hubbard, D., Ghandehari, H., & Brayden, D. J. (2014). Transepithelial Transport of PAMAM Dendrimers across Isolated Rat Jejunal Mucosae in Ussing Chambers. Biomacromolecules, 15(8), 2889–2895. http://doi.org/10.1021/bm5004465
Jung, E. S., Park, J., Gee, H. Y., Jung, J., Noh, S. H., Lee, J.-S., … Lee, M. G. (2014). Shank2 mutant mice display a hypersecretory response to cholera toxin. The Journal of Physiology, 592(8), 1809–21. http://doi.org/10.1113/jphysiol.2013.268631
Ko, E.-A., Jin, B.-J., Namkung, W., Ma, T., Thiagarajah, J. R., & Verkman, A. S. (2014). Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut, 63(7), 1120–9. http://doi.org/10.1136/gutjnl-2013-305663
Lomasney, K. W., Cryan, J. F., & Hyland, N. P. (2014). Converging effects of a Bifidobacterium and Lactobacillus probiotic strain on mouse intestinal physiology. AJP: Gastrointestinal and Liver Physiology, 307(2), G241–G247. http://doi.org/10.1152/ajpgi.00401.2013
Lomasney, K. W., Houston, A., Shanahan, F., Dinan, T. G., Cryan, J. F., & Hyland, N. P. (2014). Selective influence of host microbiota on cAMP-mediated ion transport in mouse colon. Neurogastroenterology & Motility, 26(6), 887–890. http://doi.org/10.1111/nmo.12328
Markov, A. G., Falchuk, E. L., Kruglova, N. M., Rybalchenko, O. V., Fromm, M., & Amasheh, S. (2014). Comparative analysis of theophylline and cholera toxin in rat colon reveals an induction of sealing tight junction proteins. Pflügers Archiv - European Journal of Physiology, 466(11), 2059–2065. http://doi.org/10.1007/s00424-014-1460-z
Meenach, S. A., Anderson, K. W., Hilt, J. Z., McGarry, R. C., & Mansour, H. M. (2014). High-Performing Dry Powder Inhalers of Paclitaxel DPPC/DPPG Lung Surfactant-Mimic Multifunctional Particles in Lung Cancer: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. AAPS PharmSciTech, 15(6), 1574–1587. http://doi.org/10.1208/s12249-014-0182-z
Nguyen, D. P., & Lin, S.-C. (2014). A frontal cortex event-related potential driven by the basal forebrain. eLife, 3, e02148. http://doi.org/10.7554/elife.02148
Perathoner, S., Daane, J. M., Henrion, U., Seebohm, G., Higdon, C. W., Johnson, S. L., … Levin, M. (2014). Bioelectric Signaling Regulates Size in Zebrafish Fins. PLoS Genetics, 10(1), e1004080. http://doi.org/10.1371/journal.pgen.1004080
San Martín, A., Ceballo, S., Baeza-Lehnert, F., Lerchundi, R., Valdebenito, R., Contreras-Baeza, Y., … Barros, L. F. (2014). Imaging mitochondrial flux in single cells with a FRET sensor for pyruvate. PloS One, 9(1), e85780. http://doi.org/10.1371/journal.pone.0085780
Sandler, N., Kassamakov, I., Ehlers, H., Genina, N., Ylitalo, T., & Haeggstrom, E. (2014). Rapid interferometric imaging of printed drug laden multilayer structures. Scientific Reports, 4, 4020. http://doi.org/10.1038/srep04020
Suntornsaratoon, P., Kraidith, K., Teerapornpuntakit, J., Dorkkam, N., Wongdee, K., Krishnamra, N., & Charoenphandhu, N. (2014). Pre-suckling calcium supplementation effectively prevents lactation-induced osteopenia in rats. American Journal of Physiology - Endocrinology and Metabolism, 306(2).
Tradtrantip, L., Ko, E.-A., Verkman, A. S., Walker, C., Rudan, I., Liu, L., … Shen, H. (2014). Antidiarrheal Efficacy and Cellular Mechanisms of a Thai Herbal Remedy. PLoS Neglected Tropical Diseases, 8(2), e2674. http://doi.org/10.1371/journal.pntd.0002674
Turnbull, L., Strauss, M. P., Liew, A. T. F., Monahan, L. G., Whitchurch, C. B., & Harry, E. J. (2014). Super-resolution Imaging of the Cytokinetic Z Ring in Live Bacteria Using Fast 3D-Structured Illumination Microscopy (f3D-SIM). Journal of Visualized Experiments, (91), e51469–e51469. http://doi.org/10.3791/51469
Vajn, K., Suler, D., Plunkett, J. A., Oudega, M., Becker, C., Lieberoth, B., … Umeda, K. (2014). Temporal Profile of Endogenous Anatomical Repair and Functional Recovery following Spinal Cord Injury in Adult Zebrafish. PLoS ONE, 9(8), e105857. http://doi.org/10.1371/journal.pone.0105857
Wagley, S., Hemsley, C., Thomas, R., Moule, M. G., Vanaporn, M., Andreae, C., … Titball, R. W. (2014). The twin arginine translocation system is essential for aerobic growth and full virulence of Burkholderia thailandensis. Journal of Bacteriology, 196(2), 407–16. http://doi.org/10.1128/JB.01046-13
Wang, Y., Tong, J., Chang, B., Wang, B., Zhang, D., & Wang, B. (2014). Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Molecular Medicine Reports. http://doi.org/10.3892/mmr.2014.2126
Welling, S. H., Hubálek, F., Jacobsen, J., Brayden, D. J., Rahbek, U. L., & Buckley, S. T. (2014). The role of citric acid in oral peptide and protein formulations: Relationship between calcium chelation and proteolysis inhibition. European Journal of Pharmaceutics and Biopharmaceutics, 86(3), 544–551. http://doi.org/10.1016/j.ejpb.2013.12.017
Xue, N., Li, X., Bertulli, C., Li, Z., Patharagulpong, A., Sadok, A., & Huang, Y. Y. S. (2014). Rapid patterning of 1-D collagenous topography as an ECM protein fibril platform for image cytometry. PloS One, 9(4), e93590. http://doi.org/10.1371/journal.pone.0093590
Yao, M., Goult, B. T., Chen, H., Cong, P., Sheetz, M. P., & Yan, J. (2014). Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Scientific Reports, 4, 4610. http://doi.org/10.1038/srep04610
Yusef, Y. R., Thomas, W., & Harvey, B. J. (2014). Estrogen increases ENaC activity via PKCδ signaling in renal cortical collecting duct cells. Physiological Reports, 2(5).
Zhang, J., Jiang, D., & Peng, H.-X. (2014). A pressurized filtration technique for fabricating carbon nanotube buckypaper: Structure, mechanical and conductive properties. Microporous and Mesoporous Materials, 184, 127–133. http://doi.org/10.1016/j.micromeso.2013.10.012
Zhou, Y., Chu, W., Lei, M., Li, J., Du, W., & Zhao, C. (2014). Application of a continuous intrinsic dissolution–permeation system for relative bioavailability estimation of polymorphic drugs. International Journal of Pharmaceutics, 473(1), 250–258. http://doi.org/10.1016/j.ijpharm.2014.07.012
Treating diseases mediated by blockade of the epithelial sodium channel with pyrazine-2-carboxamide derivatives. (2014).
Addis, R. C., Ifkovits, J. L., Pinto, F., Kellam, L. D., Esteso, P., Rentschler, S., … Gearhart, J. D. (2013). Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. Journal of Molecular and Cellular Cardiology, 60, 97–106. http://doi.org/10.1016/j.yjmcc.2013.04.004
Atherton, J. F., Menard, A., Urbain, N., & Bevan, M. D. (2013). Short-term depression of external globus pallidus-subthalamic nucleus synaptic transmission and implications for patterning subthalamic activity. The Journal of Neuroscience?: The Official Journal of the Society for Neuroscience, 33(17), 7130–44. http://doi.org/10.1523/JNEUROSCI.3576-12.2013
Badique, F., Stamov, D. R., Davidson, P. M., Veuillet, M., Reiter, G., Freund, J.-N., … Anselme, K. (2013). Directing nuclear deformation on micropillared surfaces by substrate geometry and cytoskeleton organization. Biomaterials, 34(12), 2991–3001. http://doi.org/10.1016/j.biomaterials.2013.01.018
Bahnemann, J., Rajabi, N., Fuge, G., Barradas, O., Müller, J., Pörtner, R., & Zeng, A.-P. (2013). A New Integrated Lab-on-a-Chip System for Fast Dynamic Study of Mammalian Cells under Physiological Conditions in Bioreactor. Cells, 2(2), 349–360. http://doi.org/10.3390/cells2020349
Birngruber, T., Ghosh, A., Perez-Yarza, V., Kroath, T., Ratzer, M., Pieber, T. R., & Sinner, F. (2013). Cerebral open flow microperfusion: A new in vivo technique for continuous measurement of substance transport across the intact blood-brain barrier. Clinical and Experimental Pharmacology and Physiology, 40(12), 864–871. http://doi.org/10.1111/1440-1681.12174
Borges, E., Setti, A. S., Vingris, L., Figueira, R. de C. S., Braga, D. P. de A. F., & Iaconelli, A. (2013). Intracytoplasmic morphologically selected sperm injection outcomes: the role of sperm preparation techniques. Journal of Assisted Reproduction and Genetics, 30(6), 849–54. http://doi.org/10.1007/s10815-013-9989-x
Brunner, E. D. (2013). Katalog der Deutschen Nationalbibliothek. Deutsche Nationalbibliothek. Retrieved from https://portal.dnb.de/opac.htm?method=simpleSearch&cqlMode=true&query=idn%253D1033270903
Choi, C. H. J., Hao, L., Narayan, S. P., Auyeung, E., & Mirkin, C. A. (2013). Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proceedings of the National Academy of Sciences of the United States of America, 110(19), 7625–30. http://doi.org/10.1073/pnas.1305804110
Cui, W., Zhang, J., Zhang, C.-X., Jiao, G.-Z., Zhang, M., Wang, T.-Y., … Tan, J.-H. (2013). Control of spontaneous activation of rat oocytes by regulating plasma membrane Na+/Ca2+ exchanger activities. Biology of Reproduction, 88(6), 160. http://doi.org/10.1095/biolreprod.113.108266
Dalby-Brown, W., Jessen, C., Hougaard, C., Jensen, M. L., Jacobsen, T. A., Nielsen, K. S., … Jørgensen, S. (2013). Characterization of a novel high-potency positive modulator of Kv7 channels. European Journal of Pharmacology, 709(1), 52–63. http://doi.org/10.1016/j.ejphar.2013.03.039
De Vos, A., Van de Velde, H., Bocken, G., Eylenbosch, G., Franceus, N., Meersdom, G., … Verheyen, G. (2013). Does intracytoplasmic morphologically selected sperm injection improve embryo development? A randomized sibling-oocyte study. Human Reproduction (Oxford, England), 28(3), 617–26. http://doi.org/10.1093/humrep/des435
Dietmann, A., Millonig, A., Combes, V., Couraud, P.-O., Kachlany, S. C., & Grau, G. E. (2013). Effects of Aggregatibacter actinomycetemcomitans leukotoxin on endothelial cells. Microbial Pathogenesis, 61–62, 43–50. http://doi.org/10.1016/j.micpath.2013.05.001
Dietmann, A., Millonig, A., Combes, V., Couraud, P.-O., Kachlany, S. C., & Grau, G. E. (2013). Effects of Aggregatibacter actinomycetemcomitans leukotoxin on endothelial cells. Microbial Pathogenesis, 61, 43–50. http://doi.org/10.1016/j.micpath.2013.05.001
Fernández, I. J., Gómez, P. N., Parodi, J., Mejía, F. R., & Salazar, R. S. (2013). Chilean crude extract of Ruta graveolens generates vasodilatation in rat aorta at subtoxic cellular concentrations. Advances in Bioscience and Biotechnology, 4(1), 29–36. http://doi.org/10.4236/abb.2013.41005
Ferreira, D. S., Reis, R. L., Azevedo, H. S., Aida, T., Meijer, E. W., Stupp, S. I., … Bröcker, E. B. (2013). Peptide-based microcapsules obtained by self-assembly and microfluidics as controlled environments for cell culture. Soft Matter, 9(38), 9237. http://doi.org/10.1039/c3sm51189h
Furtado, J. M., Ashander, L. M., Mohs, K., Chipps, T. J., Appukuttan, B., Smith, J. R., … Chiu, F. (2013). Toxoplasma gondii Migration within and Infection of Human Retina. PLoS ONE, 8(2), e54358. http://doi.org/10.1371/journal.pone.0054358
Geraldo, S., Simon, A., & Vignjevic, D. M. (2013). Revealing the Cytoskeletal Organization of Invasive Cancer Cells in 3D. Journal of Visualized Experiments, (80), e50763–e50763. http://doi.org/10.3791/50763
Hatanaka, Y., & Yamauchi, K. (2013). Excitatory cortical neurons with multipolar shape establish neuronal polarity by forming a tangentially oriented axon in the intermediate zone. Cerebral Cortex (New York, N.Y.?: 1991), 23(1), 105–13. http://doi.org/10.1093/cercor/bhr383
Henkels, J., Oh, J., Xu, W., Owen, D., Sulchek, T., & Zamir, E. (2013). Spatiotemporal mechanical variation reveals critical role for rho kinase during primitive streak morphogenesis. Annals of Biomedical Engineering, 41(2), 421–32. http://doi.org/10.1007/s10439-012-0652-y
Herricks, T., Avril, M., Janes, J., Smith, J. D., & Rathod, P. K. (2013). Clonal variants of Plasmodium falciparum exhibit a narrow range of rolling velocities to host receptor CD36 under dynamic flow conditions. Eukaryotic Cell, 12(11), 1490–8. http://doi.org/10.1128/EC.00148-13
Hosny, N. A., Mohamedi, G., Rademeyer, P., Owen, J., Wu, Y., Tang, M.-X., … Kuimova, M. K. (2013). Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proceedings of the National Academy of Sciences of the United States of America, 110(23), 9225–30. http://doi.org/10.1073/pnas.1301479110
Huda, R., McCrimmon, D. R., & Martina, M. (2013). pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. Journal of Neurophysiology.
Huda, R., McCrimmon, D. R., & Martina, M. (2013). pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. Journal of Neurophysiology, 110(2).
Jiao, G.-Z., Cao, X.-Y., Cui, W., Lian, H.-Y., Miao, Y.-L., Wu, X.-F., … Maleszewski, M. (2013). Developmental Potential of Prepubertal Mouse Oocytes Is Compromised Due Mainly to Their Impaired Synthesis of Glutathione. PLoS ONE, 8(3), e58018. http://doi.org/10.1371/journal.pone.0058018
Kim, Y., Pourgholami, M. H., Morris, D. L., Lu, H., & Stenzel, M. H. (2013). Effect of shell-crosslinking of micelles on endocytosis and exocytosis: acceleration of exocytosis by crosslinking. Biomater. Sci., 1(3), 265–275. http://doi.org/10.1039/C2BM00096B
Lancaster, O. M., Le Berre, M., Dimitracopoulos, A., Bonazzi, D., Zlotek-Zlotkiewicz, E., Picone, R., … Baum, B. (2013). Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Developmental Cell, 25(3), 270–83. http://doi.org/10.1016/j.devcel.2013.03.014
Li, X., Uchida, M., Alpar, H. O., & Mertens, P. (2013). Biolistic transfection of human embryonic kidney (HEK) 293 cells. Methods in Molecular Biology (Clifton, N.J.), 940, 119–32. http://doi.org/10.1007/978-1-62703-110-3_10
Licko, T., Seeger, N., Zellinger, C., Russmann, V., Matagne, A., & Potschka, H. (2013). Lacosamide treatment following status epilepticus attenuates neuronal cell loss and alterations in hippocampal neurogenesis in a rat electrical status epilepticus model. Epilepsia, 54(7), 1176–1185. http://doi.org/10.1111/epi.12196
Ma, X., Wang, X., Zhou, M., & Fei, H. (2013). A mitochondria-targeting gold-peptide nanoassembly for enhanced cancer-cell killing. Advanced Healthcare Materials, 2(12), 1638–43. http://doi.org/10.1002/adhm.201300037
Moeendarbary, E., Valon, L., Fritzsche, M., Harris, A. R., Moulding, D. A., Thrasher, A. J., … Charras, G. T. (2013). The cytoplasm of living cells behaves as a poroelastic material. Nature Materials, 12(3), 253–261. http://doi.org/10.1038/nmat3517
Mongkolchaipak, S., & Vutyavanich, T. (2013). No difference in high-magnification morphology and hyaluronic acid binding in the selection of euploid spermatozoa with intact DNA. Asian Journal of Andrology, 15(3), 421–4. http://doi.org/10.1038/aja.2012.163
Oulad Ben Taib, N., & Manto, M. (2013). Trains of epidural DC stimulation of the cerebellum tune corticomotor excitability. Neural Plasticity, 2013(10), 613197. http://doi.org/10.1155/2013/613197
Rouer, M., Meilhac, O., Delbosc, S., Louedec, L., Pavon-Djavid, G., Cross, J., … Alsac, J.-M. (2013). A New Murine Model of Endovascular Aortic Aneurysm Repair. Journal of Visualized Experiments, (77), e50740–e50740. http://doi.org/10.3791/50740
Saade, C. J., Alvarez-Delfin, K., & Fadool, J. M. (2013). Rod photoreceptors protect from cone degeneration-induced retinal remodeling and restore visual responses in zebrafish. The Journal of Neuroscience?: The Official Journal of the Society for Neuroscience, 33(5), 1804–14. http://doi.org/10.1523/JNEUROSCI.2910-12.2013
Saade, C. J., Alvarez-Delfin, K., & Fadool, J. M. (2013). Rod Photoreceptors Protect from Cone Degeneration-Induced Retinal Remodeling and Restore Visual Responses in Zebrafish. Journal of Neuroscience, 33(5).
Scarano, W., Duong, H. T. T., Lu, H., De Souza, P. L., & Stenzel, M. H. (2013). Folate conjugation to polymeric micelles via boronic acid ester to deliver platinum drugs to ovarian cancer cell lines. Biomacromolecules, 14(4), 962–75. http://doi.org/10.1021/bm400121q
Schroder, E. A., Lefta, M., Zhang, X., Bartos, D. C., Feng, H.-Z., Zhao, Y., … Delisle, B. P. (2013). The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. American Journal of Physiology - Cell Physiology, 304(10).
Silberberg, Y. R., & Pelling, A. E. (2013). Quantification of intracellular mitochondrial displacements in response to nanomechanical forces. Methods in Molecular Biology (Clifton, N.J.), 991, 185–93. http://doi.org/10.1007/978-1-62703-336-7_18
Sonner, P. M., & Ladle, D. R. (2013). Early postnatal development of GABAergic presynaptic inhibition of Ia proprioceptive afferent connections in mouse spinal cord. Journal of Neurophysiology, 109(8).
Suraniti, E., Vajrala, V. S., Goudeau, B., Bottari, S. P., Rigoulet, M., Devin, A., … Arbault, S. (2013). Monitoring metabolic responses of single mitochondria within poly(dimethylsiloxane) wells: study of their endogenous reduced nicotinamide adenine dinucleotide evolution. Analytical Chemistry, 85(10), 5146–52. http://doi.org/10.1021/ac400494e
Syvänen, S., Russmann, V., Verbeek, J., Eriksson, J., Labots, M., Zellinger, C., … Potschka, H. (2013). [11C]quinidine and [11C]laniquidar PET imaging in a chronic rodent epilepsy model: Impact of epilepsy and drug-responsiveness. Nuclear Medicine and Biology, 40(6), 764–775. http://doi.org/10.1016/j.nucmedbio.2013.05.008
Tonurist, K., Thomberg, T., Janes, A., & Lust, E. (2013). Specific Performance of Electrical Double-Layer Capacitors Based on Different Separator Materials and Non-Aqueous Electrolytes. ECS Transactions, 50(43), 181–189. http://doi.org/10.1149/05043.0181ecst
Torres-Mapa, M. L., Gardner, J., Bradburn, H., King, J., Dholakia, K., & Gunn-Moore, F. (2013). Femtosecond optical transfection as a tool for genetic manipulation of human embryonic stem cells. In A. Heisterkamp, P. R. Herman, M. Meunier, & S. Nolte (Eds.), SPIE LASE (p. 861104). International Society for Optics and Photonics. http://doi.org/10.1117/12.2003739
Wang, J. T.-W., Berg, K., Høgset, A., Bown, S. G., & MacRobert, A. J. (2013). Photophysical and photobiological properties of a sulfonated chlorin photosensitiser TPCS(2a) for photochemical internalisation (PCI). Photochemical & Photobiological Sciences?: Official Journal of the European Photochemistry Association and the European Society for Photobiology, 12(3), 519–26. http://doi.org/10.1039/c2pp25328c
Weinert, S., Poitz, D. M., Auffermann-Gretzinger, S., Eger, L., Herold, J., Medunjanin, S., … Braun-Dullaeus, R. C. (2013). The lysosomal transfer of LDL/cholesterol from macrophages into vascular smooth muscle cells induces their phenotypic alteration. Cardiovascular Research, 97(3), 544–52. http://doi.org/10.1093/cvr/cvs367
Weinert, S., Poitz, D. M., Auffermann-Gretzinger, S., Eger, L., Herold, J., Medunjanin, S., … Braun-Dullaeus, R. C. (2013). The lysosomal transfer of LDL/cholesterol from macrophages into vascular smooth muscle cells induces their phenotypic alteration. Cardiovascular Research, 97(3).
Yazejian, B., Yazejian, R. M., Einarsson, R., & Grinnell, A. D. (2013). Simultaneous Pre- and Post-synaptic Electrophysiological Recording from Xenopus Nerve-muscle Co-cultures. Journal of Visualized Experiments, (73), e50253–e50253. http://doi.org/10.3791/50253
Yin, B., Kuranov, R. V, McElroy, A. B., Kazmi, S., Dunn, A. K., Duong, T. Q., & Milner, T. E. (2013). Dual-wavelength photothermal optical coherence tomography for imaging microvasculature blood oxygen saturation. Journal of Biomedical Optics, 18(5), 56005. http://doi.org/10.1117/1.JBO.18.5.056005
Yuseff, M. I., & Lennon-Dumenil, A. M. (2013). Studying MHC class II presentation of immobilized antigen by B lymphocytes. Methods in Molecular Biology (Clifton, N.J.), 960, 529–43. http://doi.org/10.1007/978-1-62703-218-6_39
Zander, N. E., Orlicki, J. A., Rawlett, A. M., & Beebe, T. P. (2013). Electrospun polycaprolactone scaffolds with tailored porosity using two approaches for enhanced cellular infiltration. Journal of Materials Science: Materials in Medicine, 24(1), 179–187. http://doi.org/10.1007/s10856-012-4771-7
Zhang, J., Jiang, D., Peng, H.-X., & Qin, F. (2013). Enhanced mechanical and electrical properties of carbon nanotube buckypaper by in situ cross-linking. Carbon, 63, 125–132. http://doi.org/10.1016/j.carbon.2013.06.047
Zhu, Z., Sierra, A., Burnett, C. M.-L., Chen, B., Subbotina, E., Koganti, S. R. K., … Zingman, L. V. (2013). Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads. The Journal of General Physiology, 143(1).
Brenowitz, S. D., & Regehr, W. G. (2012). Presynaptic imaging of projection fibers by in vivo injection of dextran-conjugated calcium indicators. Cold Spring Harbor Protocols, 2012(4), 465–71. http://doi.org/10.1101/pdb.prot068551
Goel, M., Sienkiewicz, A. E., Picciani, R., Wang, J., Lee, R. K., & Bhattacharya, S. K. (2012). Cochlin, Intraocular Pressure Regulation and Mechanosensing. PLoS ONE, 7(4), e34309. http://doi.org/10.1371/journal.pone.0034309
Grama, A., & Engert, F. (2012). Direction selectivity in the larval zebrafish tectum is mediated by asymmetric inhibition. Frontiers in Neural Circuits, 6, 59. http://doi.org/10.3389/fncir.2012.00059
Huber-Reggi, S. P., Chen, C.-C., Grimm, L., Straumann, D., Neuhauss, S. C. F., & Huang, M. Y.-Y. (2012). Severity of Infantile Nystagmus Syndrome-Like Ocular Motor Phenotype Is Linked to the Extent of the Underlying Optic Nerve Projection Defect in Zebrafish belladonna Mutant. Journal of Neuroscience, 32(50).
Nakaya, N., Sultana, A., Lee, H.-S., & Tomarev, S. I. (2012). Olfactomedin 1 interacts with the Nogo A receptor complex to regulate axon growth. The Journal of Biological Chemistry, 287(44), 37171–84. http://doi.org/10.1074/jbc.M112.389916
Owen, J., Zhou, B., Rademeyer, P., Tang, M.-X., Pankhurst, Q., Eckersley, R., & Stride, E. (2012). Understanding the structure and mechanism of formation of a new magnetic microbubble formulation. Theranostics, 2(12), 1127–39. http://doi.org/10.7150/thno.4307
Seo, J., Yun, C.-O., Kwon, O.-J., Choi, E.-J., Song, J.-Y., Choi, I., & Cho, K.-H. (2012). A proteoliposome containing apolipoprotein A-I mutant (V156K) enhances rapid tumor regression activity of human origin oncolytic adenovirus in tumor-bearing zebrafish and mice. Molecules and Cells, 34(2), 143–8. http://doi.org/10.1007/s10059-012-2291-4
Boccaccio, A., Sagheddu, C., & Menini, A. (2011). Flash Photolysis of Caged Compounds in the Cilia of Olfactory Sensory Neurons. Journal of Visualized Experiments, (55), e3195–e3195. http://doi.org/10.3791/3195
Cho, K.-H. (2011). Enhanced delivery of rapamycin by V156K-apoA-I high-density lipoprotein inhibits cellular proatherogenic effects and senescence and promotes tissue regeneration. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 66(12), 1274–85. http://doi.org/10.1093/gerona/glr169
Kizil, C., & Brand, M. (2011). Cerebroventricular microinjection (CVMI) into adult zebrafish brain is an efficient misexpression method for forebrain ventricular cells. PloS One, 6(11), e27395. http://doi.org/10.1371/journal.pone.0027395
Li, W., Janardhan, A. H., Fedorov, V. V, Sha, Q., Schuessler, R. B., & Efimov, I. R. (2011). Low-energy multistage atrial defibrillation therapy terminates atrial fibrillation with less energy than a single shock. Circulation. Arrhythmia and Electrophysiology, 4(6), 917–25. http://doi.org/10.1161/CIRCEP.111.965830
Lombardi, M. L., Zwerger, M., & Lammerding, J. (2011). Biophysical Assays to Probe the Mechanical Properties of the Interphase Cell Nucleus: Substrate Strain Application and Microneedle Manipulation. Journal of Visualized Experiments, (55), e3087–e3087. http://doi.org/10.3791/3087
Seo, J. H., Jang, I. K., Kim, H., Yang, M. S., Lee, J. E., Kim, H. E., … Cho, S.-R. (2011). Early Immunomodulation by Intravenously Transplanted Mesenchymal Stem Cells Promotes Functional Recovery in Spinal Cord Injured Rats. Cell Medicine, 2(2), 55–67. http://doi.org/10.3727/215517911X582788
Cianciolo Cosentino, C., Roman, B. L., Drummond, I. A., & Hukriede, N. A. (2010). Intravenous microinjections of zebrafish larvae to study acute kidney injury. Journal of Visualized Experiments?: JoVE, (42). http://doi.org/10.3791/2079
Detrich, H. W., Westerfield, M., & Zon, L. I. (2010). Methods in cell biology. Volume 100, The zebrafish, cellular and developmental biology, part A. Academic Press.
Jala, V. R., & Haribabu, B. (2010). Real-time Imaging of Leukotriene B4 Mediated Cell Migration and BLT1 Interactions with β-arrestin. Journal of Visualized Experiments, (46), e2315–e2315. http://doi.org/10.3791/2315
Khuon, S., Liang, L., Dettman, R. W., Sporn, P. H. S., Wysolmerski, R. B., & Chew, T.-L. (2010). Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. Journal of Cell Science, 123(3), 431–440. http://doi.org/10.1242/jcs.053793
Kinkel, M. D., Eames, S. C., Philipson, L. H., & Prince, V. E. (2010). Intraperitoneal Injection into Adult Zebrafish. Journal of Visualized Experiments, (42), e2126–e2126. http://doi.org/10.3791/2126
Pelkonen, A., Hiltunen, M., Kiianmaa, K., & Yavich, L. (2010). Stimulated dopamine overflow and alpha-synuclein expression in the nucleus accumbens core distinguish rats bred for differential ethanol preference. Journal of Neurochemistry, no-no. http://doi.org/10.1111/j.1471-4159.2010.06844.x
Russek-Blum, N., Nabel-Rosen, H., & Levkowitz, G. (2010). Two-photon-based photoactivation in live zebrafish embryos. Journal of Visualized Experiments?: JoVE, (46). http://doi.org/10.3791/1902
Zou, J., & Wei, X. (2010). Transplantation of GFP-expressing Blastomeres for Live Imaging of Retinal and Brain Development in Chimeric Zebrafish Embryos. Journal of Visualized Experiments, (41), e1924–e1924. http://doi.org/10.3791/1924
Calcraft, P. J., Ruas, M., Pan, Z., Cheng, X., Arredouani, A., Hao, X., … Zhu, M. X. (2009). NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature, 459(7246), 596–600. http://doi.org/10.1038/nature08030
Gutscher, M., Sobotta, M. C., Wabnitz, G. H., Ballikaya, S., Meyer, A. J., Samstag, Y., & Dick, T. P. (2009). Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. The Journal of Biological Chemistry, 284(46), 31532–40. http://doi.org/10.1074/jbc.M109.059246
Mitra-Ganguli, T., Vitko, I., Perez-Reyes, E., & Rittenhouse, A. R. (2009). Orientation of palmitoylated CaVbeta2a relative to CaV2.2 is critical for slow pathway modulation of N-type Ca2+ current by tachykinin receptor activation. The Journal of General Physiology, 134(5), 385–96. http://doi.org/10.1085/jgp.200910204
Saha, T., Rih, J. K., & Rosen, E. M. (2009). BRCA1 down-regulates cellular levels of reactive oxygen species. FEBS Letters, 583(9), 1535–43. http://doi.org/10.1016/j.febslet.2009.04.005
3,5-diamino-6-chloro-pyrazine-2-carboxylic acid derivatives and their use as epithelial sodium channel blockers for the treatment of airway diseases. (2009).
Foust, A. J., Schei, J. L., Rojas, M. J., & Rector, D. M. (2008). In vitro and in vivo noise analysis for optical neural recording. Journal of Biomedical Optics, 13(4), 44038. http://doi.org/10.1117/1.2952295
Schei, J. L., McCluskey, M. D., Foust, A. J., Yao, X.-C., & Rector, D. M. (2008). Action potential propagation imaged with high temporal resolution near-infrared video microscopy and polarized light. NeuroImage, 40(3), 1034–43. http://doi.org/10.1016/j.neuroimage.2007.12.055
Spitler, K. M., & Gothard, K. M. (2008). A removable silicone elastomer seal reduces granulation tissue growth and maintains the sterility of recording chambers for primate neurophysiology. Journal of Neuroscience Methods, 169(1), 23–6. http://doi.org/10.1016/j.jneumeth.2007.11.026
Foust, A. J., & Rector, D. M. (2007). Optically teasing apart neural swelling and depolarization. Neuroscience, 145(3), 887–99. http://doi.org/10.1016/j.neuroscience.2006.12.068
Kopeika, J., Zhang, T., & Rawson, D. (2006). Zebrafish embryos (Danio rerio) using microinjection. Cryo Letters, 27(5), 319–28. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17256065
D’Ambrosio, R., Fairbanks, J. P., Fender, J. S., Born, D. E., Doyle, D. L., & Miller, J. W. (2004). Post-traumatic epilepsy following fluid percussion injury in the rat. Brain, 127(2).
Yavich, L., & Tiihonen, J. (2000). Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. European Journal of Pharmacology, 401(3), 365–73. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10936495
Kelly, S. M., & Macklem, P. T. (1991). Direct measurement of intracellular pressure. The American Journal of Physiology, 260(3 Pt 1), C652-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2003586
RelatedItems

AUTOLCI-BF10
AutoLCI, Brightfield, 10X Magnification Automated Live Cell Imaging System

AUTOLCI-BF4
AutoLCI, Brightfield, 4X Magnification Automated Live Cell Imaging System

AUTOLCI-BFGF10
AutoLCI, Brightfield & Green Fluorescence, 10X Magnification Automated Live Cell...

AUTOLCI-BFRF4
AutoLCI, Brightfield & Red Fluorescence, 4X Magnification Automated Live Cell Im...

AUTOLCI-BFGF4
AutoLCI, Brightfield & Green Fluorescence, 4X Magnification Automated Live Cell ...

AUTOLCI-BFRF10
AutoLCI, Brightfield & Red Fluorescence, 10X Magnification Automated Live Cell I...







Request
Catalogue
Chat
Print