

IPA
Integrated Patch Clamp Amplifier with Data Acquisition System
- Overview
- Specifications
- Accessories
- Citations
- Related Products
Overview


There are 3 images available to view - click to enlarge and scroll through the product gallery.
IPA Brochure
/ Download as PDF
Comparison Amplifier systems
/ Download as PDF
Comparison Amplifier systems
/ Download as PDF
FEATURES
- Fully integrated patch clamp amplifier and data acquisition system ensures quick and easy setup
- Optimized for whole-cell patch clamp recordings in tissue slices, adherent or dissociated cells
- Full computer control provides automated compensation of electrode and whole cell capacitance
- Voltage and current clamp capability for complete characterization of cells' electrical activity
- Bundled SutterPatch® software excels in comprehensive data management, intuitive navigation and streamlined data analysis
- Line frequency reduction in SutterPatch
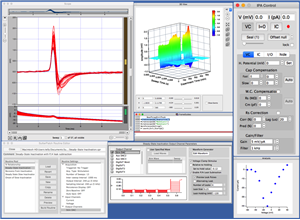
Sutter Instrument introduces the IPA® family of Integrated Patch Amplifiers, which enables efficient, low-noise whole-cell recordings. The IPA system, available with one (IPA) or two recording channels (Double IPA), combines state-of-the-art amplifier technology with fully integrated D/A and A/D conversion and a high speed USB interface. Acquisition, data management, and streamlined analysis are performed using the bundled SutterPatch® Data Acquisition and Analysis Software, built on the foundation of Igor Pro 7 (WaveMetrics, Inc.).
External Inputs & Outputs
External signals, such as environmental parameters or stimulus information, can be recorded using 4 auxiliary analog input channels. The IPA system also supports the control of peripheral hardware, such as wavelength or solution switchers, with 2 analog and 8 digital (TTL) output channels. As an alternative to the standard breakout cable, an optional Patch Panel provides a tidy way of connecting auxiliary signals on the front of your rack.
SutterPatch® Software
The IPA system, in combination with SutterPatch software, has been engineered to automatically capture and store all amplifier settings, stimulus information and external experiment parameters and associate them in time with the raw data traces. This includes all amplifier and acquisition settings, as well as timing and progress of the experiment. Fully integrated computer control of the amplifier stages means that the acquisition software is aware of the internal state of the amplifier and digitizer at all times and can track any changes that may occur. This is independent of whether a change is triggered automatically or initiated by the user
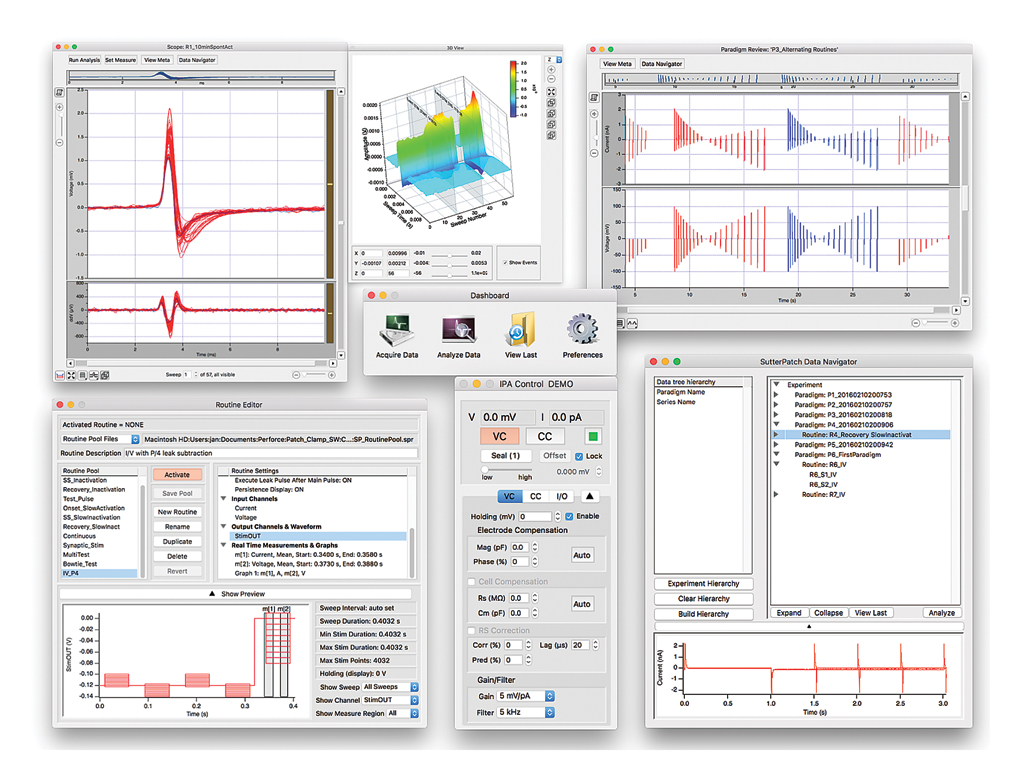
Tracking of Other External Data
In addition to status changes in connected hardware that are automatically tracked, the experimenter can manually trigger tags to document events like stimulus application in instruments not connected to the IPA system.
Information about environmental parameters and a more detailed specification of sample properties, can be recorded and stored with the raw data. A total of over 500 metadata attributes are supported. Examples include: animal species, strain, date/time when a cell sample was prepared, recording solutions, pipette resistance, hardware properties, and detailed information about stimuli applied.
Data Visualization and Analysis
SutterPatch software has been designed to simplify the navigation and analysis of complex datasets. The scope window supports multiple view modes in both two-dimensional and an innovative three-dimensional display. The 3D view is particularly useful during assay development. Built on top of the latest version of the proven Igor Pro platform, the SutterPatch program combines native Igor Pro functionality with a wealth of features that are tailored to electrophysiology applications. Both the newcomer and experienced user of patch clamp programs will feel comfortable using SutterPatch software.
Application modules provide focused functionality for particular applications.
Currently available:
- Event Detection Module: A deconvolution algorithm that excels at detecting miniature synaptic events even on a noisy background
- Camera Module: An easy way to document the identity and condition of the recorded cell
Whole-cell recordings in both voltage and current clamp experiments can easily be performed on the IPA system.
COMMON APPLICATIONS IPA
- Tissue slice recordings
- Cultured cell experiments
- Cell line studies from adherent or dispersed cells
- In-vivo patch clamp
- Network studies
- Optogenetics
Optional IPA Patch Panel
 The IPA and Double IPA Amplifiers come standard with an "octopus" breakout cable for auxiliary inputs and outputs, and digital outputs. The optional IPA Patch Panel, machined from ½" thick billet aluminum stock like the IPA faceplate, brings the auxiliary I/O connections to the front of the rack in a tidy 2U rack mount panel with BNC connectors. The IPA Patch Panel includes a 2.5 ft (76cm) connector cable and replaces the standard cable that ships with the IPA system.
The IPA and Double IPA Amplifiers come standard with an "octopus" breakout cable for auxiliary inputs and outputs, and digital outputs. The optional IPA Patch Panel, machined from ½" thick billet aluminum stock like the IPA faceplate, brings the auxiliary I/O connections to the front of the rack in a tidy 2U rack mount panel with BNC connectors. The IPA Patch Panel includes a 2.5 ft (76cm) connector cable and replaces the standard cable that ships with the IPA system.
•Fully integrated patch clamp amplifier and data acquisition system ensures quick and easy setup
•Optimized for whole-cell patch clamp recordings in tissue slices, adherent or dissociated cells
•Full computer control provides automated compensation of electrode and whole cell capacitance
•Voltage and current clamp capability for complete characterization of cells' electrical activity
•Bundled SutterPatch® software excels in comprehensive data management, intuitive navigation and streamlined data analysis
•Line frequency reduction in SutterPatch
Specifications
TECHNICAL SPECIFICATIONS
The IPA Integrated Patch Clamp Amplifier is a computer-controlled single headstage amplifier optimized for whole cell recording applications.
Amplifier
- 500 MΩ headstage feedback resistor provides a max. range of ±20 nA
- Fast and slow capacitance compensation and whole-cell compensation
- Fast compensation up to 25 pF; Slow compensation to 2.5 pF
- Whole-cell compensation: Cm from 1-100 pF ; Rs from 1-100 MΩ
- Automatic compensation routines
- Series resistance prediction and correction (0-100 MΩ)
- Four-pole Bessel low-pass filter (cutoff = 0.5 – 20 kHz)
- Output gain : 1,2,5,10,20,50,100
- Holding potential ±1000 mV
- Current injection ±20 nA
- Current clamp bridge compensation and capacitance neutralization
- Slow holding potential tracking compensates for drift during current clamp recordings
Data Acquisition
- Embedded data acquisition system eliminates the need for an external data acquisition board
- Single high-speed USB connection controls data acquisition and amplifier
- Up to 300 kHz aggregate sampling rate
- Up to 6 input channels (1 - 50 kHz sampling rate per channel)
- Holding potential command (1-10 kHz sampling rate per channel)
- Auxiliary input / output for control of other instrumentation
4 analog input channels (+/- 10V)
2 analog output channels (+/- 10V)
8 digital output channels (TTL) - Data acquisition can be initiated by an onboard microsecond clock or external (TTL) trigger
SutterPatch™ Software
- Built on the foundation of Igor Pro 7 (WaveMetrics, Inc.)
- Comprehensive data analysis routines and publication quality graphics
- Routine editor provides complete experimental control
- Associated metadata stores all relevant information regarding your experiment
- Requires Windows 7 or later (Mac OS X support pending)
Accessories
Citations
¦Aimino MA, Coker CR, Silberman Y (2018) Acute ethanol modulation of neurocircuit function in the nucleus of the tractus solitarius. Brain Res Bull 138:5-11. doi:10.1016/j.brainresbull.2017.07.019
¦ Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. (2019) Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol Stress 10:100152. doi:10.1016/j.ynstr.2019.100152
¦ Avelar AJ, Akers AT, Baumgard ZJ, Cooper SY, Casinelli GP, Henderson BJ (2019) Why flavored vape products may be attractive: Green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function. J Neuropharm 158:107729. doi:10.1016/j.neuropharm.2019.107729
¦ Doncheck EM, Anderson EM, Konrath CD, Liddiard GT, DeBaker MC, Urbanik LA, Hearing MC, Mantsch JR (2021): Estradiol Regulation of the Prelimbic Cortex and the Reinstatement of Cocaine Seeking in Female Rats. J Neurosci 41(24):5303-5314. doi:10.1523/JNEUROSCI.3086-20.2021
¦ Driscoll JR, Wallace TL, Mansourian KA, Martin WJ, Margolis EB (2020): Differential Modulation of Ventral Tegmental Area Circuits by the Nociceptin/Orphanin FQ System. eNeuro 7(5):ENEURO.0376-19.2020. doi:10.1523/ENEURO.0376-19.2020
¦ Feng T, Alicea C, Pham V, Kirk A, Pieraut S (2021): Experience-Dependent Inhibitory Plasticity Is Mediated by CCK+ Basket Cells in the Developing Dentate Gyrus. J Neurosci 41(21):4607-4619. doi:10.1523/JNEUROSCI.1207-20.2021
¦ Hu H, Wang X, Li C, Li Y, Hao J, Zhou Y,Yang X, Chen P, Shen X, Zhang S (2021): Loss of Dysbindin Implicates Synaptic Vesicle Replenishment Dysregulation as a Potential Pathogenic Mechanism in Schizophrenia. Neurosci 452:138-152. doi:10.1016/
¦ Hurley MM, Anderson EM Chen C, Maunze B, Hess EM, Block ME, Patel N, Cooper Z, McCoy R, Dabra T, Conley W, Reilly MJ, Hearing M, Choi S (2019) Acute Blockade of PACAP-Dependent Activity in the Ventromedial Nucleus of the Hypothalamus Disrupts Leptin-Induced Behavioral and Molecular Changes in Rats. Neuroendocrinol 110:271-281. doi:10.1159/000501337
¦ Hutto RA, Bisbach CM, Abbas F, Brock DC, Cleghorn WM, Parker ED, Bauer BH, Ge W, Vinberg F, Hurley JB, Brockerhoff SE (2019) Increasing Ca2+ in photoreceptor mitochondria alters metabolites, accelerates photoresponse recovery, and reveals adaptations to mitochondrial stress. Cell Death Differ 27:1067-1085. doi:10.1038/s41418-019-0398-2
¦ Madayag AC, Gomez D, Anderson EM, Ingebretson AE, Thomas MJ, Hearing MC (2019). Cell-type and region-specific nucleus accumbens AMPAR plasticity associated with morphine reward, reinstatement, and spontaneous withdrawal. Brain Struct Funct 224:2311. doi:10.1007/s00429-019-01903-y
¦ McDevitt DS, Jonik B, Graziane NM (2019) Morphine differentially alters the synaptic and intrinsic properties of D1R- and D2R-expressing medium spiny neurons in the nucleus accumbens. Front Synaptic Neurosci 11:35. doi:10.3389/fnsyn.2019.00035
¦ McGovern DJ, Polter AM, Root DH (2021): Neurochemical Signaling of Reward and Aversion to Ventral Tegmental Area Glutamate Neurons. J Neurosci 41(25):5471-5486. doi:10.1523/JNEUROSCI.1419-20.2021
¦ Nissenkorn A, Almog Y, Adler I, Safrin M, Brusel M, Marom M, Bercovich S, Yakubovich D, Tzadok M, Ben-Zeev B, Rubinstein M (2019) In vivo, in vitro and in silico correlations of four de novo SCN1A missense mutations. PLoS ONE 14(2):e0211901. doi:10.1371/journal.pone.0211901
¦ Oda K, Vierock J, Oishi S, Rodriguez-Rozada S, Taniguchi R, Yamashita K, Wiegert JS, Nishizawa T, Hegemann P, Nureki O (2018) Crystal structure of the red light-activated channelrhodopsin Chrimson. Nat Commun 9(1):3949. doi:10.1038/s41467-018-06421-9
¦ Oppermann J, Fischer P, Silapetere A, Liepe B, Rodriguez-Rozada S, Flores-Uribe J, Peter E, Keidel A, Vierock J, Kaufmann J, Broser M, Luck M, Bartl F, Hildebrandt P, Wiegert JS, Béjà O, Hegemann P, Wietek J (2019) MerMAIDs: a family of metagenomically discovered marine anion-conducting and intensely desensitizing channelrhodopsins. Nat Commun 10(1):3315. doi:10.1038/s41467-019-11322-6
¦ Qneibi M, Jaradat N, Emwas N (2019) Effect of Geraniol and Citronellol Essential Oils on the Biophysical Gating Properties of AMPA Receptors. Appl Sci 9(21):4693. doi:10.3390/app9214693
¦ Qneibi M, Hamed O, Natsheh AR, Jaradat N, Emwas N, AbuHasan Q, Al-Kerm R (2019) Inhibition and assessment of the biophysical gating properties of GluA2 and GluA2/A3 AMPA receptors using curcumin derivatives. PLoS One 14(8):e0221132. doi:10.1371/journal.pone.0221132
¦ Qneibi M, Jaradat N, Hawash M, Olac A, Emwas N (2020): Ortho versus Meta Chlorophenyl-2,3-Benzodiazepine Analogues: Synthesis, Molecular Modeling, and Biological Activity as AMPAR Antagonists. ACS Omega 5(7):3588. doi:10.1021/acsomega.9b04000
¦ Qneibi M, Jaradat NA, Hawash M, Zaid AN, Natsheh AR, Yousef R, AbuHasan Q (2019) The Neuroprotective Role of Origanum syriacum L. and Lavandula dentata L. Essential Oils through Their Effects on AMPA Receptors. Biomed Res Int 2019:1-11. doi:10.1155/2019/5640173
¦ Soma S, Yoshida J, Kato S, Takahashi Y, Nonomura S, Sugimura YK, Ríos A, Kawabata M, Kobayashi K, Kato F, Sakai Y, Isomura Y (2019) Ipsilateral-Dominant Control of Limb Movements in Rodent Posterior Parietal Cortex. J Neurosci 39(3):485. doi:10.1523/JNEUROSCI.1584-18.2018
¦ Vierock J, Rodriguez-Rozada S, Dieter A, Pieper F, Sims R, Tenedini F, Bergs ACF, Bendifallah I, Zhou F, Ahlbeck J, Augustin S, Sauter K, Papagiakoumou E, Soba P, Emiliani V, Engel AK, Hegemann P, Zeitzschel N, Gottschalk A, Wiegert JS (2021): BiPOLES is an optogenetic tool developed for bidirectional dual-color control of neurons. Nature Comm 12:2547. doi:10.1038/s41467-021-24759-5
¦ Wietek J, Rodriguez-Rozada S, Tutas J, Tenedini F, Grimm C, Oertner TG, Soba P, Hegemann P, Wiegert JS (2017) Anion-conducting channelrhodopsins with tuned spectra and modified kinetics engineered for optogenetic manipulation of behavior.
Sci Rep 7:14957. doi:10.1038/s41598-017-14330-y
RelatedItems

MPC-385
One MP-285 manipulator mechanical, one MPC-200 controller, one ROE-200, mounting ada...









Request
Catalogue
Chat
Print